By
David Bautz, PhD
NASDAQ:CERC
CERC-301 Misses Primary Endpoint in Phase 2 Trial in Major Depressive Disorder
On November 29, 2016, Cerecor, Inc. (NASDAQ:CERC) announced topline results for the Phase 2 study of CERC-301 in major depressive disorder (MDD). The results showed that the trial failed to show efficacy on the primary endpoint for mean improvement in the Bech-6 scale, which is a subset of the Hamilton Depression Scale (HDRS-17), averaged over Days 2 and 4 post dose. While the result of the trial was disappointing, the company did report a potentially clinically meaningful effect on the Bech-6 and HDRS-17 for the 20 mg dose group on Day 2.
The Phase 2 trial was a randomized, double blind, placebo controlled study evaluating the antidepressant effect of 12 mg and 20 mg doses of CERC-301 in MDD patients currently experiencing a severe depressive episode despite stable ongoing treatment with either an SSRI or SNRI (NCT02459236). A total of 115 patients were randomized into the trial, which was conducted over two sequential one-week periods. In Period 1, 21 patients each were randomized to receive a 12 mg or 20 mg dose of CERC-301 while 72 patients were administered placebo. Assessments were taken on Days 2 and 4 following dosing. In Period 2, those who received CERC-301 received another dose, while those who received placebo in Period 1 were divided into responders (n=23) and non-responders (n=49). The placebo responders again received another dose of placebo. The non-responders were then randomized to receive either a 12 mg or 20 mg dose of CERC-301 or placebo. Assessments were taken on Days 2 and 4 following administration of the second dose of treatment. This is depicted in the following graphic.
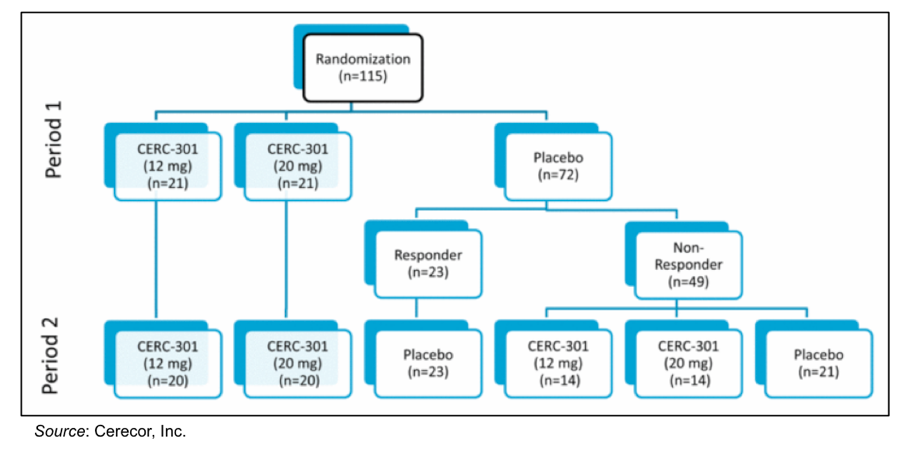
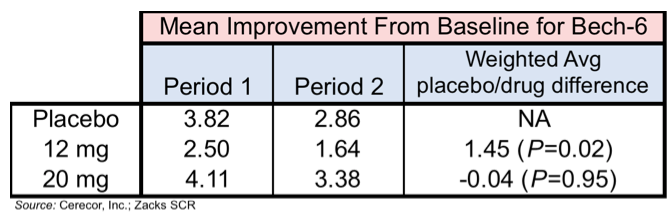
The following chart shows the results in the mean Bech-6 Averaged over Days 2 and 4 for Period 1 and 2 along with the weighted average for the difference between placebo and drug. The placebo dose was statistically significantly better than the 12 mg dose, while there was no statistical difference between the 20 mg dose and placebo.
The one positive that could be taken from this trial in regards to a potential efficacy signal is from the CERC-301 20 mg group on Day 2 following dosage for both Bech-6 and the HDRS-17. The following table shows that the mean improvement from baseline favored the 20 mg group for each of the measurements, however none of the results was statistically significant due to the small number of patients in each group.
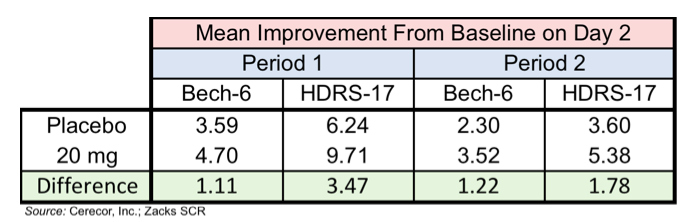
It should be noted that the differences seen in HDRS-17 between the 20 mg group and placebo on Day 2 compare favorably with both Abilify and Rexulti, two adjunctive antidepressant medications approved by the FDA. Treatment with Abilify resulted in a difference from placebo of 2.5 on HDRS-17 (Berman et al., 2009) while treatment with Rexulti resulted in a difference from placebo of 2.29 on HDRS-17 (Thase et al., 2015).
Safety and tolerability of CERC-301 was quite good, as no serious adverse events were reported. The most commonly reported adverse events were increased blood pressure, dizziness, somnolence, and paresthesia. Since CERC-301 was so well tolerated, it may make sense for the company to conduct future studies with a higher dose of drug and/or increase the frequency of dosing from once a week to two or more times per week. However, future development plans for CERC-301 will not be known until the company has a chance to go over the full data set and meet with the FDA to discuss any additional clinical studies.
Focus Turns to CERC-501
Cerecor is developing CERC-501, an orally available, highly specific kappa opioid receptor (KOR) antagonist, as a treatment for substance use disorder, adjunctive treatment of MDD, and potentially for co-occurring disorders. CERC-501 is highly selective for the KOR as it has 21-fold higher affinity for the KOR compared to the mu receptor and 135-fold higher affinity over the human delta opioid receptor (Mitch et al., 2011). Preclinical studies showed that CERC-501 dose-dependently produced an antidepressant-like response in the forced swim test and significantly attenuated continuous ethanol self-administration in female rats with a history of high ethanol intake (Rorick-Kehn et al., 2014).
The company is currently conducting the Clin501-201 clinical trial, which is a randomized, double blind, placebo controlled trial to evaluate the effects of 15 mg of CERC-501 on tobacco withdrawal and reinstatement and to assess craving, mood, and anxiety during 18 hours of abstinence in 66 heavy cigarette smokers (NCT02641028). Cerecor received a $1 million grant from the National Institute on Drug Abuse to help fund the study. We anticipate results in December 2016. The company has stated that it does not intend to continue development of CERC-501 for smoking withdrawal at this time, thus the most important data to come out of the current study is likely to be the safety and tolerability of the drug.
Cerecor is currently planning a Phase 2/3 trial of CERC-501 in adjunctive MDD, which would likely initiate in the second half of 2017. Validation for the use of a KOR antagonist as an adjunctive treatment to antidepressant therapy in MDD comes from recently released data from a Phase 3 study (FORWARD-5) conducted by Alkermes plc (ALKS) for ALKS-5461, which is a combination drug formulation of buprenorphine and samidorphan. FORWARD-5 was a randomized, double blind, placebo controlled, sequential parallel comparison design study that enrolled 407 patients with MDD who had an inadequate response to a stable dose of a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI). The results showed that ALKS 5461 2mg/2mg met the primary endpoint of significant reducing depression scores compared to placebo, as measured by the 6-item Montgomery-Asberg Depression Rating Scale (MADRS-6) scores (P=0.018) and the 10-item MADRS (MADRS-10) scores (P=0.026). These results clearly show that a KOR antagonist can have a positive effect in treating MDD, thus we believe Cerecor is fully justified in focusing resources on this opportunity.
Conclusion and Valuation
We were disappointed that the Phase 2 trial of CERC-301 failed to reach statistical significance for the primary endpoint, however we believe the potential efficacy signals observed on Day 2 for the 20 mg dose group should be evaluated in future clinical studies. We anticipate knowing more about the future development of CERC-301 once the company has a chance to evaluate the full data set and we anticipate presentations on the data at scientific conferences during 2017.
We believe that the company will now shift its focus to CERC-501, as preparations are underway for a Phase 2/3 clinical trial in adjunctive MDD and results from the ongoing Phase 2 study in nicotine withdrawal are set to be announced in December 2016. Developing CERC-501 in adjunctive MDD makes additional sense with the positive Phase 3 data reported by Alkermes for ALKS-5461, which is also a KOR antagonist.
Based on the results of the Clin301-203 trial we have made significant adjustments to our model. We have removed CERC-301 from our valuation model, and replaced it with CERC-501 as a treatment for adjunctive MDD. We model for potential regulatory filings in 2021 and approval in 2022. Our model estimates a price of $20 per day in the U.S. and $16 per day in the rest of the world and assume that patients would take the drug for approximately nine months out of the year. We model for potential peak sales of CERC-501 in MDD of $1.8 billion worldwide. With a 40% probability of success and an 18% discount rate, we arrive at a net present value of $100 million.
Combining the net present value for CERC-501 and CERC-611 along with the company’s current cash total and expected operating burn of $30 million we arrive at a net present value for the company of $78.9 million. Dividing this by the company’s fully diluted (common stock and options) share count of approximately 11.1 million shares leads to a valuation of $7/share. Positive results next month from the CERC-501 study could cause the shares to align more closely with our valuation.
For a free copy of the full research report, please email scrinvestors@zacks.com with CERC as the subject.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR and to view our disclaimer.