NASDAQ:PIRS
Business UpdateOn September 26, 2016, Pieris Pharmaceuticals, Inc. (NASDAQ:PIRS)
announced the presentation of positive preclinical data for its lead immunooncology (IO) product PRS-343 at the 2016
CRI-CIMT-AACR International Cancer Immunotherapy Conference. Below is an overview of PRS-343 and the new preclinical data, which shows anti-tumor activity along with increased frequency of tumor-infiltrating lymphocytes in a humanized mouse tumor model.
PRS-343
PRS-343 is a bispecific compound that contains a 4-1BB(CD137)-specific Anticalin® genetically linked to a HER2-specific monoclonal antibody (trastuzumab), and is the lead compound from the company’s IO program. 4-1BB is a tumor necrosis factor receptor (TNFR) family member with costimulatory function that regulates T-cell proliferation and survival and is expressed on activated CD8+ and CD4+ T-cells (
Watts, 2005). Since CD8+ T-cells play a pivotal role in tumor immunity, enhancing 4-1BB costimulation could promote the generation of protective antitumor immune responses.
Activation of 4-1BB is known to trigger an anti-cancer response, as exhibited through enhanced immunogenicity in tumor cells engineered to express 4-1BB ligand (
Mogi et al., 2000) or anti-4-1BB antibodies (
Ye et al., 2002). 4-1BB is expressed in high levels on intratumoral T-cells (
Palazón et al., 2012). This is shown in the following graph, where CD4+ and CD8+ T-cells were extracted from the spleen, lymph nodes, and tumor of mice bearing CT26 tumors. Only T-cells isolated from tumors showed expression of 4-1BB, whereas T-cells from other locations from the same mice did not show increased expression of 4-1BB.
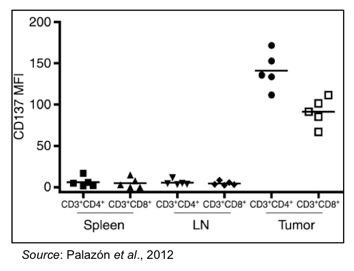
Since 4-1BB-positive T-cells are present in the tumor microenvironment, the theory behind PRS-343 is that getting an Anticalin® that can bind to and activate 4-1BB in the tumor microenvironment is likely to initiate an anti-tumor response while decreasing the risk of systemic side effects since the activated T-cells will be localized near the tumor. PRS-343 contains a “homing beacon” in the form of a HER2-specific monoclonal antibody that directs the compound to tumors that express HER2, a tumor-associated antigen that is overexpressed on a number of different tumor types. Attaching a 4-1BB-specific Anticalin® to the HER2 antibody means that once inside the tumor microenvironment, the 4-1BB-specific Anticalin® can engage with and activate 4-1BB-postive T-cells and initiate a proper anti-tumor T-cell response.
This T-cell response is generated through a “clustering” phenomenon, where binding of multiple 4-1BB molecules leads to an enhanced activation signal. This “clustering” occurs because of the overexpression of HER2 on the tumor cell surface. By bringing together the tumor cell and the T-cell, the T-cell receptor can then interact with any peptides presented on the tumor cell surface through the major histocompatibility complex (MHC), resulting in a tumor-specific T-cell response. This is all depicted in the following graphic.

Another important aspect of PRS-343 is that it is less likely to induce serious systemic side effects than other 4-1BB-specific therapies due to activation of 4-1BB only occurring in the tumor microenvironment. The following figure shows the results of a luciferase reporter assay performed with Jurkat reporter cells that overexpress 4-1BB, in which activation of the 4-1BB signaling pathway leads to an increase in luminescence. The following graphs show that PRS-343 activates the 4-1BB signaling pathway, but only in the presence of HER2-overexpressing NCI-N87 cells. In contrast, an anti-4-1BB antibody leads to activation of the 4-1BB signaling pathway regardless of whether tumor cells are present or not. These data support the idea that PRS-343 will not activate 4-1BB outside of the tumor microenvironment, as no “clustering” is likely to occur away from HER2-overexpressing cells.
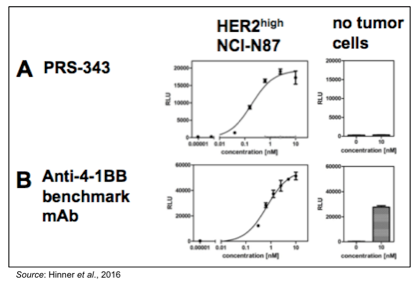
PRS-343 Shows Activity in Humanized Mouse Tumor Model
To test PRS-343 in vivo, immuno-compromised mice were engrafted with SK-OV-3 cells, which are a human ovarian cancer cell line that overexpresses HER2. When the tumors reached a volume of approximately 120 mm3, the mice were injected with human peripheral blood mononuclear cells (PBMC), which include lymphocytes (T cells, B cells, NK cells) and monocytes, along with the first dose of treatment. Treatments were given on Day 0, 7, and 14 and consisted of a 4-1BB specific monoclonal antibody, an IgG4 isotype control antibody, PRS-343 at 4 different doses, or a modified version of trastuzumab (anti-HER2 antibody) with an IgG4 backbone (to match the backbone on PRS-343).
The results of the experiment show that the benchmark 4-1BB antibody had very little effect on tumor growth with results very similar to the control isotype antibody. There was also a dose response to PRS-343, with the 4 μg and 20 μg doses having a lower anti-tumor effect than the 100 μg and 200 μg doses. The higher doses of PRS-343 resulted in approximately 50% tumor shrinkage. In addition, there was a strong anti-HER2 effect, as shown by the IgG4-trastuzumab having similar efficacy to the 100 μg and 200 μg doses of PRS-343.
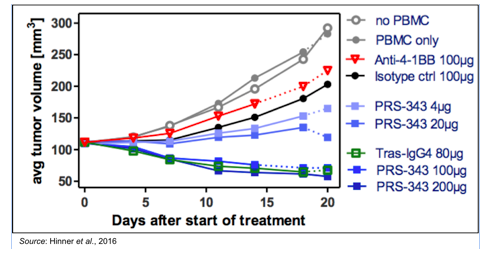
Some may see the above data and wonder why there was no difference between treatment with PRS-343 and trastuzumab, however the reason for that is likely due to the limitations of the model and dose not imply anything regarding the potential of PRS-343. The most important take away from these studies is that PRS-343 has a dual mechanism of action. It retains the ability to block HER2 pathway signaling (as shown by having similar efficacy to trastuzumab) while also having the capability to induce a robust 4-1BB mediated increase in tumor infiltrating lymphocytes (TILs; discussed below).
In order to see an anti-tumor response with PRS-343 above and beyond that which is possible through anti-HER2 mechanisms, two things are necessary: 1) having T-cells that are capable of responding to a tumor through interaction of the MHC and T-cell receptor (TCR); and 2) binding of 4-1BB to provide a co-stimulatory enhancement to TCR-mediated activity. In the model shown above, immuno-compromised mice were infused with PBMC isolated from
healthy humans. Thus, while those cells are capable of being activated through binding of 4-1BB, it is unlikely those PBMCs would be capable of mounting a TCR-mediated response as there would be few if any cancer-specific cytotoxic T-cells (CD8+). However, what the model clearly shows is that there is modulation of the tumor microenvironment, with an increase in the number of TILs.
The following figure shows enhanced staining for CD45+ cells in tumors taken from mice treated with PRS-343 with little to no enrichment of CD45+ cells in mice treated with the 4-1BB antibody or trastuzumab. CD45 is a marker for B- and T-cell activation. The conclusion from these experiments is that PRS-343 is capable of increasing the frequency of TILs. While not able to be seen in this particular model, in a patient with a HER2+ tumor the increased number of CD45+ cells are likely to contain a proportion of tumor antigen specific T-cells capable of receiving the second co-stimulatory TCR-mediated signal, leading to an enhanced anti-tumor T-cell mediated response.
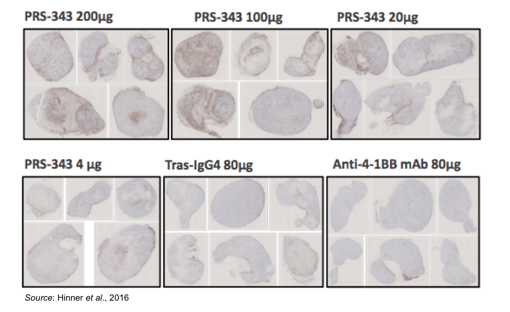
Any treatment that augments the activity of T-cells runs the risk of immune-mediated disease. However, the mechanism of action for PRS-343 is less likely to lead to systemic activation of the immune system in the periphery due to the two co-stimulatory signals required for T-cell activation. This is evident in the following figures, which show a greatly increased toxicity in mice treated with an anti-4-1BB antibody leading to increased mortality from graft-versus-host disease. This was due to a greatly increased percentage of CD45+ cells in the periphery (not in the tumor microenvironment) that contained a large percentage of CD8+ T-cells in animals treated with anti-4-1BB antibody.
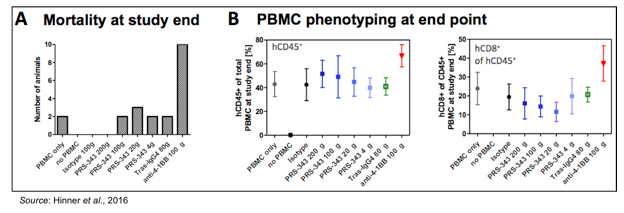
In summary, the data presented by Pieris show that PRS-343 is capable of activating 4-1BB, but only in the presence of tumor cells that overexpress HER2. This mechanism of action will likely mitigate the chances for systemic immune system activation in the periphery and likely result in a more benign safety profile than therapies that only target 4-1BB activation. In a humanized mouse model, PRS-343 leads to an increase in TILs, which should result in an enhanced anti-tumor response against HER2-expressing cancers in patients. Lastly, PRS-343 is readily expressed using industry standard mammalian expression systems (CHO cells) with a high yield and a very low level of high molecular weight species.
Conclusion
The preclinical data presented for PRS-343 is very encouraging and we are now quite interested to see how PRS-343 will respond in the clinic. We believe that Pieris will file an IND for PRS-343 and begin a clinical trial in the first half of 2017. The study is likely to involve patients with HER2-expressing tumors, thus in addition to gaining insight into the PD/PK for PRS-343, this study could give early insight into potential anti-tumor activity.
We continue to be big fans of Pieris and the Anticalin® platform and believe that the move into IO was an excellent strategic decision, as real success with IO treatments will necessitate a multi-target approach, something for which the Anticalin® platform is particularly well-suited. Our probability adjusted discounted cash flow model leads to a valuation of $8 per share, and we believe any investor interested in small cap biotech should take a serious look at Pieris.
For a free copy of the full research report, please email scrinvestors@zacks.com with PIRS as the subject.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR and to view our disclaimer.