By
David Bautz, PhD
NASDAQ:CFRX
READ THE FULL CFRX RESEARCH REPORT
Business Update
Additional Data Presented for Exebacase Phase 2 Trial
On April 16, 2019, Contrafect Corp. (NASDAQ:CFRX)
announced the presentation of new data from the Phase 2 clinical trial of exebacase (CF-301) for the treatment of
Staphylococcus aureus bacteremia including endocarditis at the 29th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). The data was presented by Vance G. Fowler, Jr., M.D., Professor of Medicine in the Division of Infectious Diseases at Duke University. The Phase 2 trial was an international, multicenter, randomized, double blind, placebo controlled trial with a superiority comparison between exebacase or placebo combined with the standard of care antibiotics. A total of 121 patients were randomized 3:2 to receive a single dose of 0.25 mg/kg exebacase or placebo administered via a two-hour infusion along with the standard of care (SOC) antibiotics. The primary endpoint of the study was early clinical response. A patient was considered a responder if they were alive, had an improvement or resolution of the signs and symptoms attributable to the
S. aureus infection, there were no additional medical interventions required, and there was no evidence of a spread of the infection. Early clinical response was determined by an independent, blinded adjudication committee. Please see our previous
report for a discussion of the topline data from the study.
The company had previously announced a 42.8% improvement in responder rate at Day 14 for patients with methicillin-resistant
S. aureus (MRSA) treated with exebacase + SOC compared to SOC alone (
P=0.010). The following figure shows new data presented at ECCMID in which clinically meaningful increases were seen in responder rate at Day 7 and test of cure (TOC) in MRSA patients treated with exebacase + SOC compared to SOC alone.
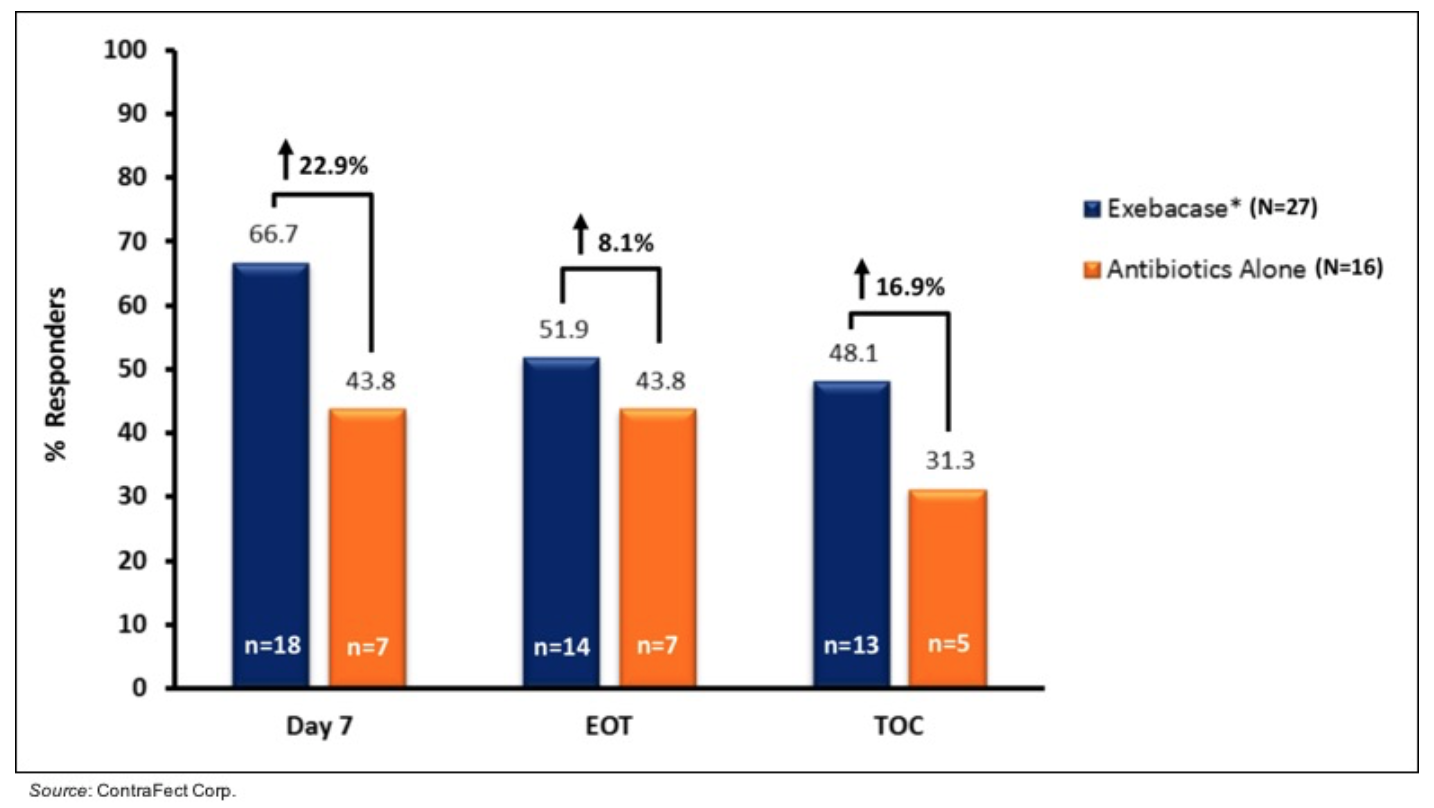 View Exhibit I
View Exhibit IOne of the largest confounding factors in the exebacase Phase 2 trial had to do with the distribution of final diagnoses between the two groups. The following figure shows that there was a large difference in patients diagnosed with left-sided endocarditis (LIE) between the two treatment groups, with 15.5% of patients in the exebacase + SOC group having LIE compared to only 6.7% in the SOC only group. This is important, as LIE is a notoriously difficult condition to treat (
Cahill et al., 2017). As an example of how difficult patients with left-sided endocarditis are to treat, in a clinical trial comparing the efficacy of daptomycin to vancomycin for the treatment of bacteremia and endocarditis caused by
S. aureus only 11% (1/9) of patients with left-sided endocarditis responded to treatment with daptomycin (
Fowler et al., 2006).
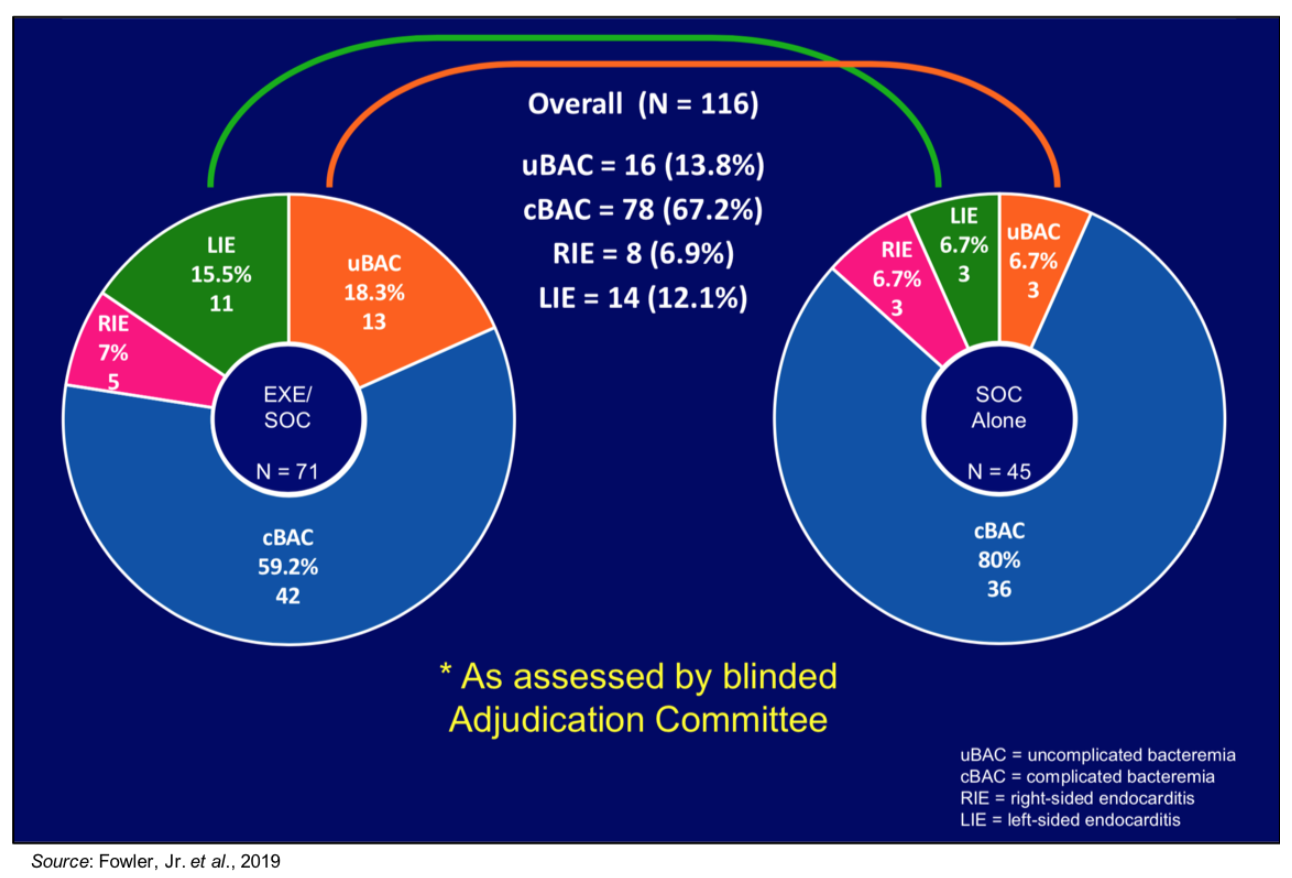 View Exhibit II
View Exhibit IIThe issue with LIE patients is exemplified by the following figure, which shows responder rates at Day 14 according to final diagnosis, which was a pre-specified secondary analysis. Only 18.2% (2/11) of patients with LIE were responders in the exebacase + SOC group. However, treatment with exebacase in patients with complicated bacteremia led to a 20.3% higher responder rate compared to patients treated only with SOC.
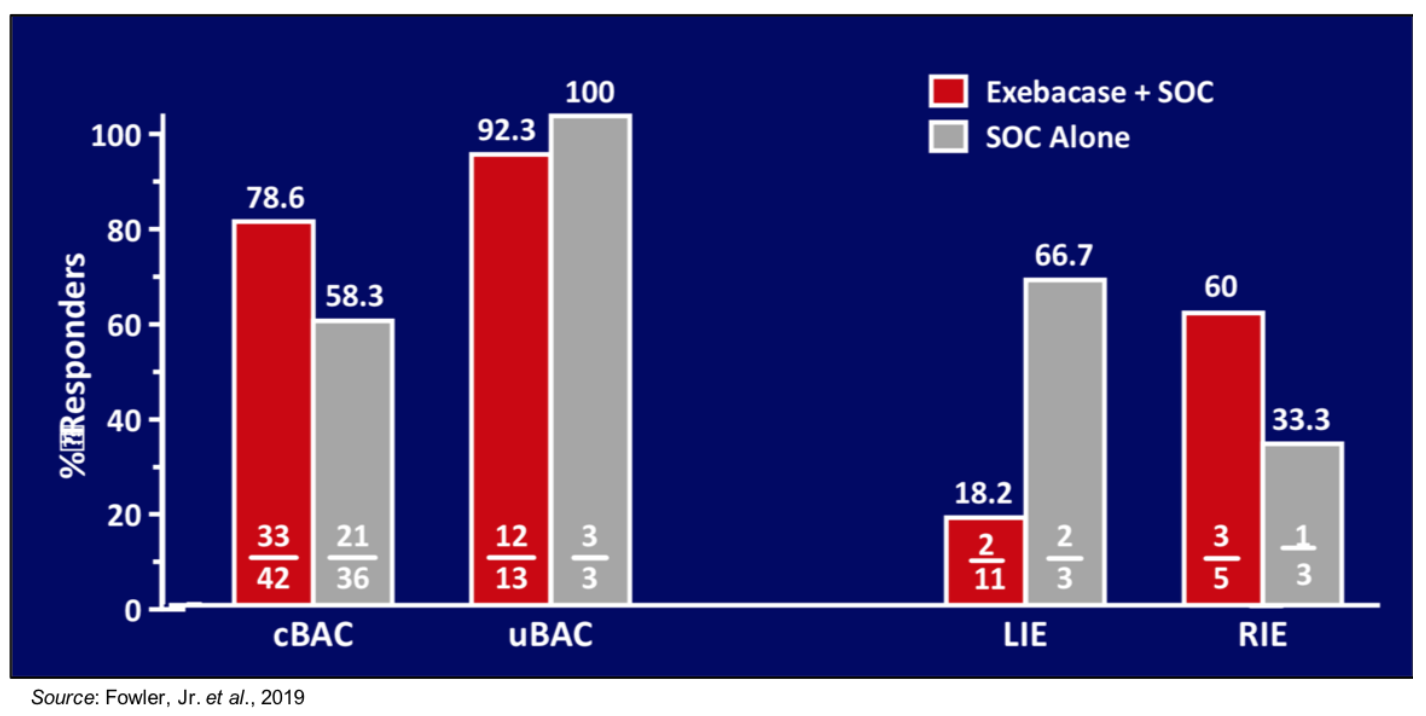 View Exhibit III
View Exhibit III As previously reported, exebacase was safe and very well tolerated. The following chart shows that the percentage of treatment-emergent adverse events (TEAE) was similar in both treatment groups and there were no reports of adverse events due to hypersensitivity to exebacase along with no serious TEAEs that were determined to be related to exebacase.
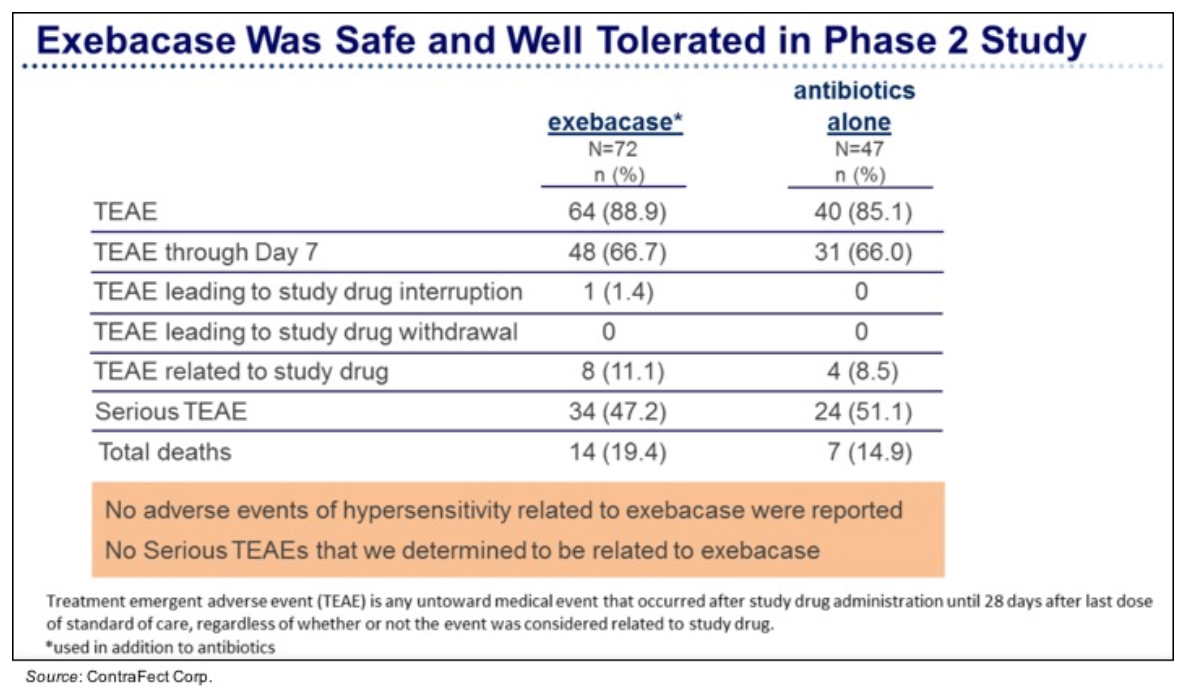 View Exhibit IV
View Exhibit IVWe believe the Phase 2 data presented by ContraFect fully supports the advancement of exebacase into a Phase 3 program. We anticipate the company conducting an ‘end-of-Phase 2’ meeting with the FDA this summer to receive regulatory guidance on a Phase 3 clinical trial, followed by a meeting with the EMA in the second half of 2019. We anticipate learning additional details about the Phase 3 program in the second half of 2019 following the company’s meeting with regulatory authorities, and anticipate a Phase 3 trial initiating in the first half of 2020.
Exebacase Synergizes with Human Blood Factors
On April 8, 2019, ContraFect
announced the publication of a manuscript titled “The Antistaphylococcal Lysin, CF-301, Activates Key Host Factors in Human Blood to Potentiate Methicillin-Resistant
Staphylococcus aureus Bacteriolysis” in the peer-review journal
Antimicrobial Agents and Chemotherapy (
Indiani et al., 2019). The paper describes the enhanced activity of exebacase in several
in vitro assays when performed in human blood compared to standard testing buffer (cation-adjusted Meuller-Hinton broth [caMHB]). The researchers identified lysozyme and human serum albumin (HSA) as two blood constituents that contribute to the enhanced activity of exebacase, which is surprising given that neither has previously been known to have antistaphylococcal activity. While the exact mechanism of this synergism is still being elucidated, this work helps to further differentiate lysins from small-molecule antibiotics that typically have reduced activity in the presence of albumin. In addition, it supports the potential use of lysins in other environments, including synovial fluid (which has similar albumin concentrations as blood) and infected bone, skin, and/or soft tissues that have significant exposure to blood components.
Leadership Change
On April 2, 2019, ContraFect
announced that Roger J. Pomerantz, MD has been appointed Chairman and Chief Executive Officer to succeed Steven Gilman, PhD, who is retiring. Dr. Pomerantz has served as Vice Chairman of ContraFect for the past five years, thus he is very familiar with the company’s technology and current position. Previously, Dr. Pomerantz was Chairman and CEO of Seres Therapeutics, where he continues to serve as the Chairman of the Board of Directors.
Conclusion
We are encouraged by the strong results from the Phase 2 trial, particularly in MRSA patients. We are perplexed by the stock’s reaction following the release of the Phase 2 results, as it was a first-in-patient study that set out to identify the proper target population for a Phase 3 program, which we believe was accomplished. Perhaps even more importantly, the results validated the lysin platform, for which ContraFect has a number of other development projects in the pipeline. We look forward to an update from the company later this year on the Phase 3 program and its initiation in the first half of 2020. Our valuation remains $4.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $30,000 annually for these services. Full Disclaimer HERE.