By John Vandermosten, CFA
EPA:ALQGC
READ THE FULL ALQGC.PA RESEARCH REPORT
REFRESH First Patient Enrolled
Quantum (EPA:ALQGC) announced on July 8, 2021 that the first patient had been enrolled in its pivotal Phase III REFRESH trial of once-daily firibastat in difficult-to-treat and resistant hypertension. REFRESH is the last step in an FDA-endorsed development plan needed before submission for market authorization in 4Q:23. The study is being conducted with partners DongWha for the South Korean market and Orient Europharma for Southeast Asia, Australia and New Zealand. The multicenter, multinational study is targeting total enrollment of 750 patients with difficult to treat or resistant hypertension, defined by persistent hypertension despite administration of two antihypertensive drugs at maximum tolerated doses and three antihypertensive drugs including a diuretic at maximum tolerated doses, respectively. Quantum anticipates participation of 96 study sites across Europe, Canada, US, Taiwan and South Korea. Efficacy results and six month safety results are expected in mid-2023.
Phase III REFRESH Launched
On January 18, 2021, Quantum announced the launch of REFRESH, a Phase III pivotal trial of once daily firibastat in difficult-to-treat1 hypertension and resistant2 hypertension, a key milestone in the pursuit of global commercialization for Quantum’s lead candidate. The study is part of an overall Phase III evaluation of firibastat. If successful, the once-daily administration will provide additional convenience and compliance compared to the twice-daily regimen being investigated in the FRESH trials. The goal of REFRESH is to assess both long-term safety and three-month efficacy in the once-daily dose. The launch of the trial will not impact the anticipated timeline for the filing of firibastat which is expected in 2023.
Dosing for REFRESH will be 1000 mg firibastat, administered once per day to individuals with treatment-resistant hypertension. The total number of target sites has not yet been determined as Quantum is still finalizing territories and associated partnerships. Some clinical sites are already being used in other Quantum trials easing site selection, while others, in new geographies, will need to be established.
For the first three months of treatment, patients in REFRESH will receive 1000 mg firibastat once-daily in addition to their current regimen. The primary endpoint is reduction in systolic automated office blood pressure from baseline. Following the three-month efficacy evaluation, treatment will continue with follow-up for six months. 100 patients will receive follow up exams at 12 months to assess long-term safety. As hypertension often requires chronic use of medication, the long-term safety data generated in REFRESH is necessary for submission of a New Drug Application (NDA).
Key Milestone for Quantum: QUORUM Study Results Announcement
Topline results for the QUORUM study will be presented at the European Society of Cardiology (ESC) Congress being held from August 27 – 30, in a virtual format. The communication of the data will take place on August 27 at 10:30 am Central European Summer Time (CEST). QUORUM (Firibastat Or Ramipril after Acute Myocardial infarction to prevent left ventricular dysfunction) is a multi-center, multinational, randomized, double-blind, active-controlled trial designed to assess the efficacy and the safety of firibastat compared to ramipril. Our valuation model attributes approximately 14% of Quantum’s valuation to success of firibastat in post-myocardial infarction heart failure (MI HF). Strong results from this trial could potentially support even greater value for post MI HF indication compared with the hypertension target given our estimates of a higher penetration rate into this smaller, but less competitive market.
Quantum representatives will also be presenting other information at ESC. On August 28th, Dr. Catherine Llorens-Cortes, one of the inventors of BAPAI, will be presenting the details of a preclinical study of QGC606 in heart failure after myocardial infarction. The title of the presentation is Comparison of QGC606, a novel orally active brain-penetrating aminopeptidase A inhibitor prodrug with firibastat and ramipril for treating heart failure following myocardial infarction, which will be offered at the Late Breaking Basic & Translational Science session.
Other Events
Since our previous update in April, Quantum has provided several updates related to partners, financing, publications and anticipated conference presentation in addition to advancing the REFRESH trial. On April 20, the company announced the end of its collaboration with Qilu in China. The two companies were not able to reconcile the objectives for firibastat development in the Chinese market and aborted their agreement to commercialize the region. Quantum has recovered development rights and is now opening discussions with other potential collaborators. No risk of litigation or penalties were identified by Quantum Genomics.
In late April, Quantum secured €3 million in non-dilutive financing from government-backed, low interest loans. Half comes from BNP at a 0.25% interest rate and half from a research and development loan from BPIfrance at a 0.72% rate. The first loan comes due in one year and the second has a seven year maturity. As of the end of 2020, Quantum held €28.5 million in cash on the balance sheet and is expected to burn about €1 million per month. The loans should extend the funding of operations by about a quarter at a very favorable cost of capital.
In May, Quantum reported the publication of a scientific article in Biomedicine & Pharmacotherapy entitled "Effects of firibastat in combination with enalapril and hydrochlorothiazide on blood pressure and vasopressin release in hypertensive DOCA-salt rats." The paper examined oral administration of firibastat in hypertensive rats. Firibastat efficacy was evaluated in combination with enalapril and hydrochlorothiazide. Administration of oral firibastat in triple combination produced a significant decrease in blood pressure whereas enalapril and hydrochlorothiazide alone had minimal impact. The triple combination also performed better than using firibastat alone. The animal study was supportive of firibastat being used in combination therapy for blood pressure control in difficult to treat or resistant patient populations.
Pipeline
Quantum’s pipeline consists of one drug, firibastat, with various formulations pursuing multiple indications. It is being investigated in difficult-to-treat and resistant hypertension, heart failure, renal failure also in once per day formulations. The candidate is currently in one Phase III trial for hypertension, one Phase II trial for heart failure and Phase I trials for QD formulation hypertension and renal failure.
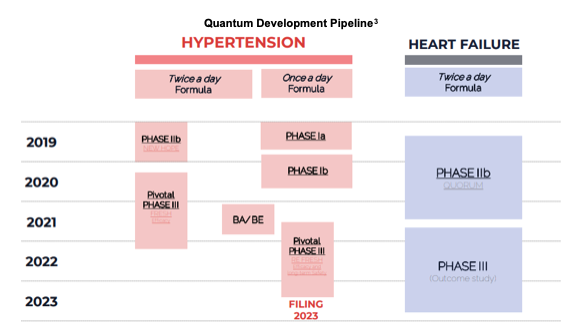
Milestones
➢ FRESH first patient enrolled – July 2020
➢ €20 million private placement – December 2020
➢ Launch REFRESH study – January 2021
➢ Orient EuroPharma Equity Stake – February 2021
➢ First patient enrolled in REFRESH study – July 2021
➢ QUORUM Phase II results, presented at ESC – August 2021
➢ FRESH study results – 4Q:21
➢ With supportive data, launch Ph3 outcome study in HF – 2021/2022
➢ Topline results for REFRESH study – Mid-year 2023
Summary
Quantum Genomics continues to make headway in the clinic, reaching its latest milestone of enrolling its first patient in the REFRESH study. Quantum is building a global market for firibastat with development and commercialization partnerships around the world. Additional opportunity remains for other deals in countries such as Japan, India, North America, China and unpenetrated areas in Europe. Despite the Qilu setback, there are plenty of other catalysts that are expected in the near future. The most prominent is the presentation of topline data from the Phase IIb QUORUM trial, which will provide proof of concept for the use of firibastat in heart failure. New sources of capital and cash reserves of almost €29 million suggest a runway beyond 2022. We expect additional funds in coming quarters which will further support development programs and perhaps obviate the need for further capital raises.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
________________________
1. Hypertension that is not controlled despite two antihypertensive classes, including a diuretic, at maximum tolerated doses.
2. Hypertension that is not controlled despite treatment with at least three antihypertensive classes, including a diuretic, at maximum tolerated doses.
3. Source: Quantum Genomics March 2021 Corporate Presentation