By David Bautz, PhD
NASDAQ:ARWR
READ THE FULL ARWR RESEARCH REPORT
Business Update
Catalyst-Rich Summer Ahead for Arrowhead
Arrowhead Pharmaceuticals Inc (NASDAQ:ARWR) is developing medicines that cause gene silencing using RNA interference (RNAi), a specific means of inhibiting the expression of genes and stopping the production of a specific protein. The company has a deep and diverse pipeline consisting of the following development product candidates, including eight in-house programs and six partnered drugs, four with Johnson and Johnson (JNJ), one with Amgen (AMGN), and one with Takeda.
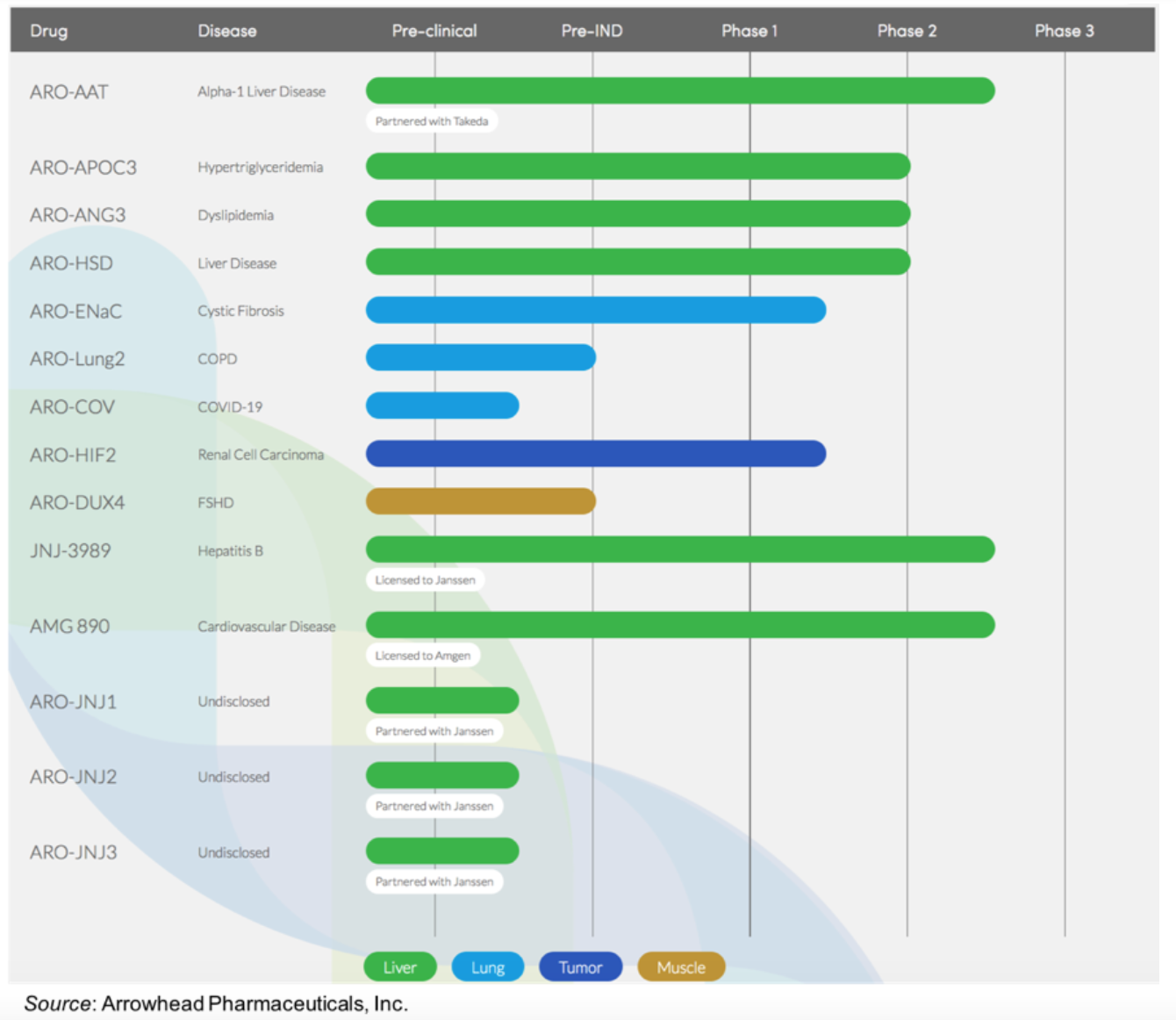
This summer, we anticipate the following catalysts:
1) Initiate two Phase 2b studies of ARO-APOC3 in patients with hypertriglyceridemia and a Phase 3 study in patients with familial chylomicronemia syndrome (FCS)
2) Initiate a Phase 2b study of ARO-ANG3 in patients with mixed dyslipidemia (elevated triglycerides and LDL cholesterol)
3) Announce interim results from the ARO-ENaC first-in-human study
4) Present full 48-week biopsy results for ARO-AAT from the AROAAT2002 open label study at a scientific conference
5) Announce interim results from the ARO-HSD first-in-human study
6) Announce initial interim results from the ARO-HIF2 first-in-human study
7) File a CTA for ARO-DUX4, the company’s recently announced first muscle-targeted RNAi candidate as a potential treatment for facioscapulohumeral muscular dystrophy
8) Announced preclinical data for ARO-DUX4 at the FSHD Society International Research Congress
9) Announce additional pulmonary programs that are in the IND-enabling stage
ARO-AAT
ARO-AAT is being developed as a treatment for the rare genetic liver disease alpha-1-antitrypsin (AAT) deficiency. This program was granted Fast Track status by the FDA in June 2019 (which was in addition to Orphan Drug designation in the U.S. and E.U. granted in early 2018). In October 2020, Arrowhead announced a collaboration with Takeda Pharmaceuticals in which the companies will co-develop ARO-AAT and co-commercialize the drug in the U.S., if approved, with a 50/50 profit-sharing structure. Arrowhead received an upfront payment of $300 million and is eligible to receive potential development, regulatory, and commercial milestones of up to $740 million. Takeda is responsible for commercialization outside the U.S. and Arrowhead is eligible to receive royalties of 20-25% on net sales.
Arrowhead is currently conducting the SEQUOIA Phase 2/3 trial and the AROAAT2002 open label study. The company recently announced interim 48-week liver biopsy results from the AROAAT2002 study.
• For cohort 2 (n=5), the results showed that 4/5 patients achieved a 1-stage or greater improvement in Metavir fibrosis stage, with no worsening of fibrosis in the fifth patient. In addition, all five patients had reductions in histological globule assessment scores. Lastly, total intra-hepatic mutant AAT protein (Z-AAT) decreased by 77-97%.
• For cohort 1 (n=4), after only 24-weeks of treatment 2/4 patients achieved a 1-stage or greater improvement in Metavir fibrosis stage (both patients who showed improvement entered the study with F4 cirrhosis). In addition, all four patients had reductions in histological globule assessment scores and total intra-hepatic Z-AAT decreased by 72-95%.
These results are incredibly encouraging and show that when Z-AAT protein is cleared the liver has the ability to heal, including in patients with severe liver disease (cirrhosis). We anticipate additional details for these results being presented at an upcoming scientific meeting. In addition, based on these results we believe the company will likely approach regulators to discuss accelerating the development of ARO-AAT.
ARO-ENaC
ARO-ENaC targets the epithelial sodium channel (ENaC) and is being developed for the treatment of cystic fibrosis (CF). CF patients have reduced clearance of dehydrated mucus due to a defect in the CFTR gene that conducts chloride ions across epithelial cell membranes. The lack of Cl- movement and continued activity of ENaC promotes the dehydration of mucus, however inhibiting the activity of ENaC improves this condition.
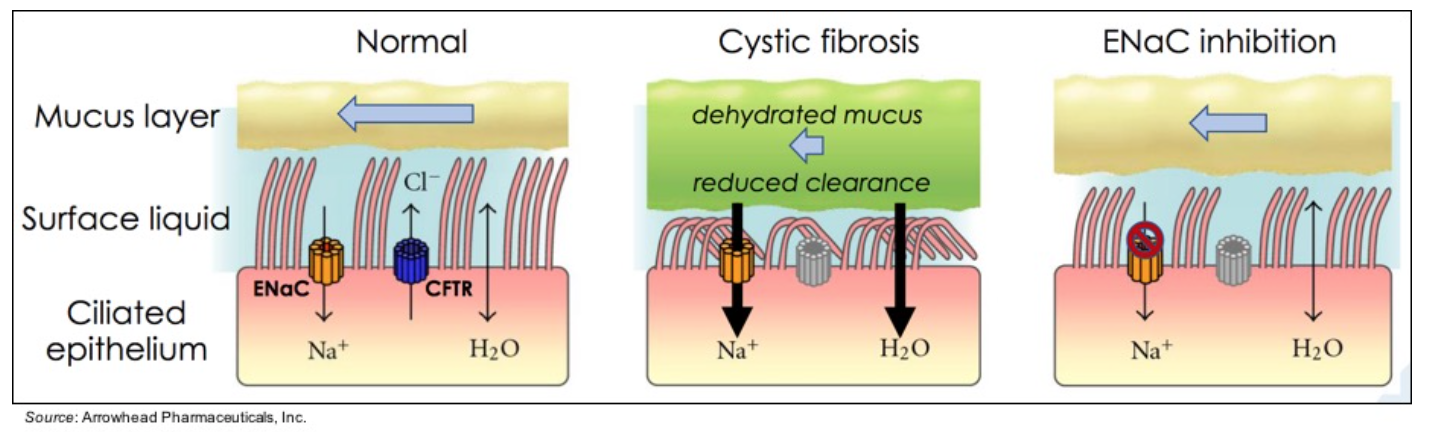
Multiple studies validate ENaC as a target in CF. A mutation that increases ENaC activity in patients with a mutation in only one CFTR allele (CFTR+/-) causes atypical CF, thus suggesting that decreased ENaC activity could decrease CF pathophysiology (Rauh et al., 2010). A loss-of-function mutation in ENaC in pseudohypoaldosteronism (PAH) results in no sodium absorption from airway surfaces, a volume of airway surface liquid that is more than twice the normal value, and an increase in mucociliary clearance compared to healthy individuals (Kerem et al., 1999). Lastly, CF patients with a homozygous F508del mutation who live into their fifth or sixth decade of life were identified and found to have mutations in ENaC genes (Agrawal et al., 2017).
Thus far, Arrowhead has shown in preclinical models that ARO-ENaC can durably silence pulmonary αENaC expression in a dose dependent manner in rats and preserves lung clearance in a sheep mucostatic model of CF. While there have been many advancements in the treatment of CF patients, opportunities still exist to help those patients that either a) don’t respond to standard of care therapies and/or b) to enhance the response for those on standard of care therapies. Importantly, treatment targeting ENaC can be used in all CF patients, regardless of genotype.
The company is currently conducting a Phase 1/2 clinical trial in 24 healthy volunteers and up to 24 CF patients. Thus far, dosing has completed in all single-dose healthy volunteers and the company is pleased with the safety and tolerability seen thus far. This is important as previous ENaC small molecule inhibitors have been dose limited by toxicity.
We anticipate FEV1 and lung clearance index (LCI) data from the first dosing levels in CF patients and ENaC knockdown data in healthy volunteers in mid-2021. Results will only be available for a small number of CF patients (n=4 in the first cohort), thus interpreting FEV1 and LCI data may be difficult from this early readout based on the high level of variability typical for those outcomes. However, ENaC knockdown in healthy volunteers should be easier to interpret. The company is hoping to see 50% knockdown because the long-lived CF patients with heterozygous mutations in ENaC (comparable to a 50% knockdown) showed a clinically meaningful benefit, however it remains to be seen what level of knockdown will be necessary to see a clinically meaningful benefit.
ARO-HIF2
ARO-HIF2 is designed to treat clear cell renal cell carcinoma (ccRCC) and targets hypoxia inducible factor 2α (HIF2α). Approximately 74,000 cases of kidney cancer were diagnosed in 2019, with approximately 70-80% of those being ccRCC. The Von Hippel-Lindau (VHL) tumor suppressor gene is inactivated in the majority of ccRCC cases. Phosphorylated VHL controls the degradation of HIFs, and numerous studies have shown that the overexpression of HIF2α is a driver of ccRCC. Thus, suppression of HIF2α may be a good target for treating ccRCC.
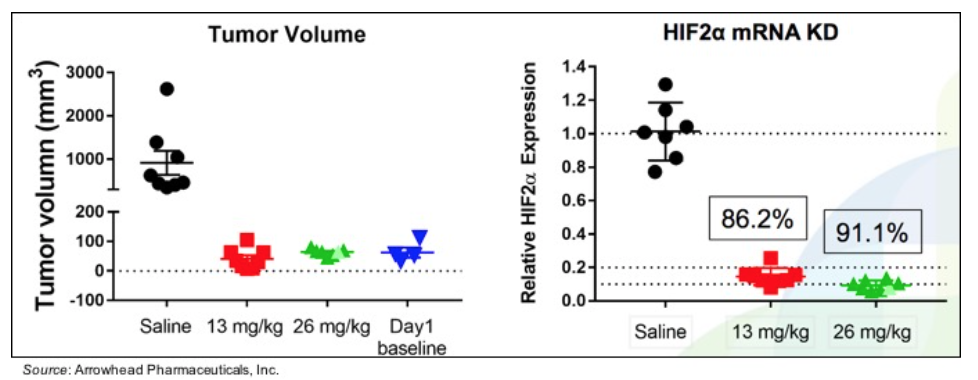
ARO-HIF2 was studied in a mouse model of ccRCC that utilizes the cell line A498, which contains a VHL mutation and overexpresses HIF2α. The following graph shows that both 13 mg/kg and 26 mg/kg ARO-HIF2 controlled tumor growth, with tumor volumes similar to what was seen on Day 1. In addition, levels of HIF2α mRNA were decreased 86.2% and 91.1% compared to vehicle control, showing excellent target engagement.
Arrowhead is currently conducting a Phase 1b dose-finding trial in three cohorts with at least six patients per cohort. Dosing has completed in two of the three cohorts. We anticipate HIF2α knockdown data based on pre- and post-treatment biopsies in mid-2021, and just as with ARO-ENaC, management believes that 50% knockdown would be a positive signal for the program. The company is also evaluating preliminary efficacy data, but it is likely too soon to derive anything meaningful on PFS or ORR outcomes.
ARO-HSD
ARO-HSD targets hydroxysteroid 17β-dehydrogenase 13 (HSD17B13), a member of the HSD17B family that is markedly upregulated in patients and mice with non-alcoholic fatty liver disease (NAFLD) (Su et al, 2019). Loss-of-function mutations in HSD17B13 provide the strongest known protection against non-alcoholic steatohepatitis (NASH) cirrhosis, alcoholic hepatitis, and cirrhosis (Abul-Husn et al., 2018). In the CDAA (choline-deficient, methionine-reduced, 60% fat) mouse model of NASH, once-weekly treatment with 3 mg/kg ARO-HSD resulted in decreased steatosis, inflammation, and hepatocyte degeneration along with inhibition of liver fibrosis.
Arrowhead is conducting a Phase 1/2 single and multiple dose-escalating study and have completed the single dose portion in healthy volunteers’ cohorts and completed dosing in two of the four multiple-dose cohorts in patients with NASH or suspected NASH. Due to the fact that HSD17B13 is not a secreted protein the only way to assess proper target engagement is through liver biopsies and we anticipate biopsy data in mid-2021. In contrast to other agents in development for NASH, we don’t anticipate any effect on liver fat, but at this point will instead be interested in the depth and duration of knockdown of HSD17B13.
ARO-DUX4
ARO-DUX4 is Arrowhead’s first muscle targeted program and is being developed for the treatment of facioscapulohumeral muscular dystrophy (FSHD). FSHD is a rare genetic disorder characterized by progressive muscle weakness and degeneration most notably in the face, shoulders, and upper arms, however it usually causes weakness in multiple muscle groups all over the body. The disease is caused by the inappropriate expression of the transcription factor double homeobox protein 4 gene (DUX4)(Gabriëls et al., 1999). Normally, hypermethylation of the end of chromosome 4 (known as D4Z4) keeps DUX4 silenced. However, in FSHD patients, hypomethylation of this region prevents DUX4 from being silenced. Aberrant expression of DUX4 dysregulates a number of different signaling pathways that culminates in cytotoxicity, particularly in muscle cells.
We anticipate the company presenting preclinical data for ARO-DUX4 in mid-2021 and filing for regulatory approval to initiate clinical studies in the third quarter of 2021. ARO-DUX4 fits with the company’s objectives to move RNAi outside of liver-specific targets and also target genes for which there is believed to be a clear cause for a specific disease.
ARO-APOC3 and ARO-ANG3
ARO-APOC3 and ARO-ANG3 are Arrowhead’s two wholly-owned cardiometabolic candidates. Please see our previous report for a discussion of the clinical trial data generated thus far for each of those programs. Over the next few months we anticipate the company initiating two Phase 2b clinical trials and a Phase 3 clinical trial for ARO-APOC3: a Phase 2b trial in patients with triglycerides >500 mg/dL, a Phase 2b trial in patients with triglycerides between 150 mg/dL and 500 mg/dL, and a Phase 3 trial in patients with familial chyolomicronemia syndrome (FCS). For ARO-ANG3, the company will be initiating a Phase 2b clinical trial in patients with elevated triglycerides and elevated LDL cholesterol. We anticipate all of those trials initiating over the next few months.
Financial Update
On May 4, 2021, Arrowhead announced financial results for the second quarter of fiscal year 2021 that ended March 31, 2021. The company reported revenue of approximately $32.8 million for the second quarter of fiscal year 2021 compared to approximately $23.5 million for the second quarter of fiscal year 2020. This revenue consists of the recognition of $25.4 million associated with the Takeda License Agreement and the recognition of a portion of the $252.7 million associated with the agreement with Janssen.
R&D expenses for the three-month period ending March 31, 2021 were $44.7 million compared to $29.4 million for the three-month period ending March 31, 2020. The increase was primarily due to increased salaries, facilities costs, and non-cash stock-based compensation. G&A expenses for the second quarter of fiscal year’s 2021 and 2020 were $16.3 million.
Arrowhead exited the second quarter of fiscal year 2021 with approximately $549.0 million in cash, cash equivalents, and investments. As of April 30, 2021, Arrowhead had approximately 104.1 million shares outstanding and, when factoring in stock options and restricted stock units, a fully diluted share count of approximately 111.7 million.
Conclusion
We look forward to the numerous data readouts, trial initiations, and presentations that are anticipated over the next few months. In particular, we will be interested in what type of knockdown ARO-ENaC is capable of, although we continue to believe there will be too few CF patients analyzed to gain any insight into changes in FEV. In addition, we look forward to the initial data from the ARO-HSD and ARO-HIF2 first-in-human studies. Our valuation for Arrowhead remains at $100, with the potential for further upside if there are positive results later this year from ARO-ENaC, ARO-HIF2, and/or ARO-HSD.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.