By John Vandermosten, CFA
NASDAQ:ECOR
READ THE FULL ECOR RESEARCH REPORT
Fourth Quarter and Full Year 2020 Financial and Operational Results
On March 11, 2021, electroCore Inc. (NASDAQ:ECOR) presented its 2020 financial and operational results in a press release and SEC filed Form 10-K. Fourth quarter revenues of $928,000 rose 38% over prior year levels, ahead of our forecasts for $875,000. While up year over year, revenues declined on a sequential basis due to a third quarter bolus in sales to the Veteran’s Administration (VA) that pulled forward demand and the impact from a surge in COVID cases during the last months of the year. Loss per share was ($0.14) in the fourth quarter, which included several non-core items. After adjustment for these items, we calculate a loss per share of ($0.11). 2020 was a big year for electroCore, despite starting off with uncertainty related to the pandemic. Revenues rose sequentially in the first second and third quarter and were up 46% for the year reaching $3.5 million. Highlights for the year include capital raises from Lincoln Park Capital, grant of emergency use authorization (EUA) for gammaCore nVNS therapy to treat COVID symptoms, the launch of multiple investigator initiated studies and further penetration into the United Kingdom’s National Health Service (NHS). Execution of a distributor agreement in Eastern Europe and results from the PREMIUM II study were announced prior to year-end. In 2021, electroCore announced the receipt of a unique Level II Healthcare Common Procedure Coding System (HCPCS) designator, additional distributor agreements, study outcomes in multiple indications and 510(k) clearance of gammaCore to treat adolescent migraine.
Since our previous update on electroCore on February 10, the company has announced additional positive news, most importantly the distribution agreement with Medistar for distribution in Australia and New Zealand, and clearance to expand gammaCore’s label to include children between 12 and 17 years old in the preventive treatment of migraine. Recent positive developments that we discussed in our prior report are also material and include advancements in the clinical work, study readouts and further penetration into existing relationships. In the clinical domain, electroCore published its study on noninvasive vagus nerve stimulation (nVNS) to reduce ileus after major colorectal surgery. electroCore also reported that the TR-VENUS study of nVNS in acute treatment of stroke was fully enrolled. In December, topline results were released for the PREMIUM II trial in migraine prevention and gammaCore was selected for a National Institute on Drug Abuse (NIDA) sponsored study in opioid use disorder. In the commercial and regulatory sphere, gammaCore Sapphire was included in the new National Health Service (NHS) England and NHS Improvement MedTech Funding Mandate Policy. Scottish Health Technology Group recommended gammaCore for use in NHS Scotland cluster headache patients and Centers for Medicare and Medicaid Services (CMS) specified coding for nVNS. Exclusive distribution rights were assigned to RSK Medical, Pro Medical Baltic and Medistar for Canada, Eastern European territories and Australia, respectively.
Fourth Quarter Financial Results
Revenues of $928,200 represented an increase of 38% over prior year levels with VA, NHS and commercial sales increases driving the change. Fourth quarter 2002 VA revenues, which comprise the largest component of total revenues, were $509,000, up 35% year over year driven by a 49% increase in VA paid months of therapy. Sales in the United Kingdom rose 25% to $281,000 on continued penetration into the NHS despite a negative impact from the pandemic. These gains were partially offset by declines in sales to Germany that at $23,000 were about half prior year levels.
Gross margin was negatively impacted by an increase in inventory reserves. Reported gross margin was 12% when the full $434,000 related to the reserves were included in the cost of goods sold. When added back, gross margin rose to a more respectable 59%. Operating expenses were $6.4 million, down 25% from prior year levels. Note that SG&A includes a $557,500 write-off related to an operating lease for the company’s previous office space. Net loss was ($6.3) million or ($0.14) per share compared with 4Q:19 net loss of ($8.9) million and ($0.30) per share.
National Health Service (NHS)
On January 27, 2021, electroCore announced that gammaCore Sapphire had been included in a new long-term reimbursement policy. The policy was launched by Matthew Whitty, Director of Innovation Research and Life Sciences and Chief Executive of the Accelerated Access Collaborative, for and on behalf of NHS England and NHS Improvement.
The new policy, called MedTech Funding Mandate Policy 2021/22 (MTFM), will go into effect on April 1, 2021. It supports the use of medical devices, diagnostics and digital technologies that will improve patient outcomes. The new policy allows gammaCore to transition away from the Innovation and Technology Payment (ITP) program that we had reported on previously, which required periodic renewal. The MTFM policy will allow sustained regional reimbursement arrangements across England, providing more certainty of long term revenues from this program. Inclusion in MTFM validates gammaCore’s efficacy, affordability and cost savings and is projected to save the NHS as much as £4.6 million over the next five years.
electroCore reported that gammaCore received a two year extension of its listing in the NHS supply chain catalog. Previously, the device was expected to be listed until June 3, 2021 and will now be extended for an additional two years through June 3, 2023. Availability in the catalog will ensure that gammaCore Sapphire is available to hospitals and listing as an e-Direct product will allow for easier and more cost effective direct delivery of gammaCore from the supplier.
Scottish Health Technology Group Recommendation for Use of gammaCore
Based on 2019 guidance by NICE, Health Improvement Scotland (HIS) published a Scottish Health Technology Group (SHTG) adaptation including gammaCore for cluster headache, announced January 21, 2021. The NICE guidance states that gammaCore plus standard of care can reduce frequency and intensity of cluster headache attacks and improve quality of life while saving costs through reduction of rescue medication use. The SHTG publication recommends gammaCore be available for 3-month trial for NHS Scotland cluster headache patients. The SHTG adaptation is now being disseminated across NHS Scotland health boards by HIS.
Establishment of Unique Level II HCPCS Code for nVNS
In the US, electroCore announced on January 19, 2021 that the CMS had published its most recent decisions regarding Level II Healthcare Common Procedure Coding System (HCPCS) on January 15, 2021. The decisions included the establishment of a unique code, K1020, for non-invasive vagus nerve stimulator, which covers gammaCore Sapphire. electroCore had submitted an application during CMS’ second biannual 2020 Coding Cycle for non-drug and non-biological items and services. The application focused on the clinical and economic benefits of gammaCore therapy. Final coding decisions will go into effect on April 1, 2021, a major milestone in obtaining additional coverage within the US medical benefits space. The availability of the code simplifies negotiations with payors due to the nVNS-specific designator in contrast to the previous regime where a miscellaneous code would be used. The old code was commonly used for transcutaneous electrical nerve stimulation (TENS) devices, which have a lower reimbursement level than gammaCore, complicating negotiations. The availability of the code will also allow for a more rapid reimbursement process and patients will be able to access therapy sooner.
Recent Distribution Deals
Exclusive Distribution Agreement with Medistar in Australia
An agreement was signed between electroCore and Medistar on February 28 for the latter to serve as the exclusive distributor of the gammaCore Sapphire non-invasive vagus nerve stimulator in Australia. Approval for the device to be marketed on the continent was recently granted by the Australian Therapeutic Goods Administration (TGA). The term of the agreement is for three years and includes minimum purchase commitments. Official launch of the device will be in mid-March at the ANZHS Headache Annual Scientific Meeting.
Exclusive Distribution Agreement with RSK Medical Inc. in Canada
electroCore signed an agreement with RSK Medical Inc. to make RSK the exclusive distributor for gammaCore Sapphire in Canada. The agreement was announced January 26, 2021. The agreement marks a milestone for commercialization in Canada. RSK Medical is headquartered in Ontario and is a medical device supplier.
Agreement with Pro Medical Baltic to be Exclusive Distributor for gammaCore Sapphire in Eastern Europe
electroCore’s agreement with Pro Medical Baltic was announced on December 21, 2020. Through the agreement, Pro Medical Baltic received exclusive distribution rights to gammaCore Sapphire for primary headache disorders in Lithuania, Latvia, Belarus, Kazakhstan and Ukraine. Pro Medical Baltic has experience with distributing another neuromodulation device, which is expected to help gammaCore accelerate its commercialization process.
We anticipate additional deals to be announced in the coming months. Some of the regions that we think are likely to be high on the priority list for electroCore include Western Europe, Middle East and Asia. Revenues from each of the abovementioned agreements should be recognized in 2021. These arrangements are particularly attractive to electroCore given the low capital requirements and minimum purchase requirements.
Corporate Milestones
electroCore has multiple approved indications and is currently pursuing additional ones with active studies in respiratory symptoms in COVID patients, stroke, post-traumatic conditions, traumatic brain injury and others which are at varying stages of advancement. Below we list recent milestones and anticipated future events.
➢ Complete enrollment for SAVIOR-1 – 1Q:21
➢ Clinical publications for PTSD, PTH & TBI – 4Q:20/1Q:21
➢ Premium II data readout – December 2020
➢ Grant of HCPCS code – January 2021
➢ EU stroke data – 1Q:21/2Q:21
➢ Distribution partner announcements outside US – Ongoing
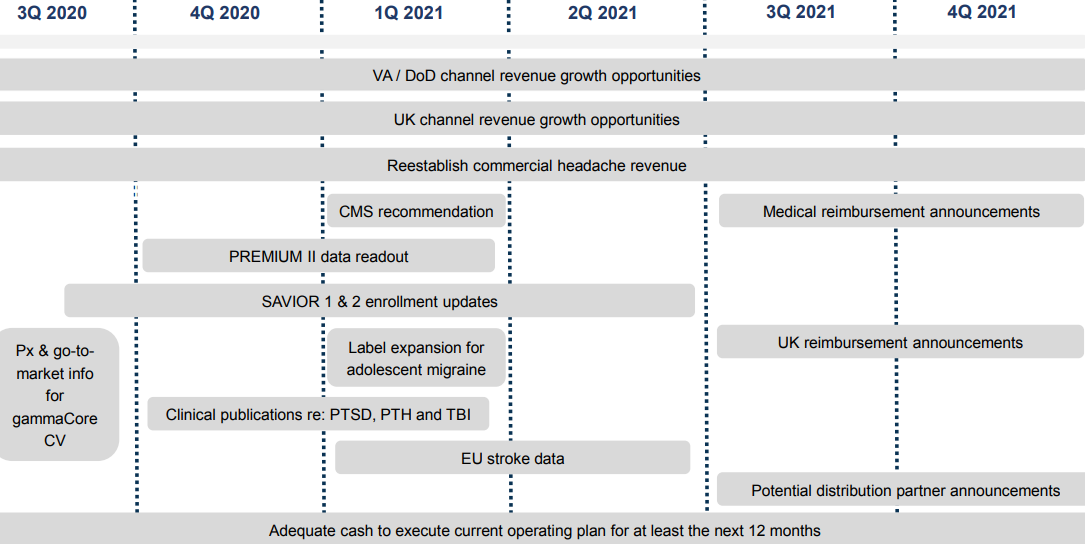
Anticipated Achievements and Milestones (1)
Summary of Investment Thesis
➢ Effective, non-invasive, and non-pharmaceutical method to treat migraines
➢ Portable, simple and rapid solution for preventing and treating headaches
➢ Indicated for both migraines and cluster headaches
➢ Existing and expanding relationships with VA hospitals
➢ Recharge/refill business model
➢ Supply chain simplicity: direct to customer shipping
➢ gammaCore can be prescribed through telemedicine
➢ NHS relationship in UK may provide foundation for further global expansion
◦ Continental Europe
◦ Asia
➢ Multiple distributor agreements with additional agreements expected
◦ Eastern Europe
◦ Canada
◦ Australia
➢ Opportunity for COVID-related sales following EUA award
➢ Extended indications
◦ Stroke
◦ Trauma injury related headaches
◦ PTSD
◦ Mild traumatic brain injury
◦ Inflammation
Summary
electroCore has impressed us with its broad array of opportunities that either the company or investigator-led activity is pursuing. A study in Turkey, examining the benefit of gammaCore in stroke patients, multiple studies by the VA, two studies in the United States and Spain investigating the benefits of gammaCore in COVID patients and other studies that are reviewing efficacy of the stimulation device in opioid use disorder and to reduce ileus following colorectal surgery are all underway and funded by entities outside of electroCore. Success has also come in the British Isles with the Innovation Technology Program Award. This program is now transitioning to the more predictable MedTech Funding Mandate and a recommendation by Health Improvement Scotland for the NHS to use gammaCore for cluster headache in Scotland. Distribution is also advancing sales efforts with new arrangements signed with Pro Medical Baltic for activities in Eastern Europe, RSK Medical in Canada and Medistar in Australia.
Updated guidance calls for first quarter revenues of approximately $1 million and cash burn of $4.5 to $4.8 million. electroCore has many factors in its favor which are helping to expand its presence globally. Additional indications are also longer term opportunities such as those in respiratory distress, traumatic headache, mild traumatic brain injury, PTSD, stroke and inflammatory diseases. The incremental positives that have emerged support our recent target price increase to $4.50 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
________________________
1. electroCore Corporate Presentation March 2021