By John Vandermosten, CFA
NASDAQ:HTBX
READ THE FULL HTBX RESEARCH REPORT
Business Update Conference Call
Heat Biologics, Inc. (NASDAQ:HTBX) hosted a business update conference call on September 2, 2021 to discuss the status of active programs, objectives of new subsidiaries, and longer term opportunities for the company. The replay for the call is available through September 16th.
New Subsidiaries: SkunkWorx and Scorpion
Heat CEO, Jeff Wolf, opened the meeting by providing an update on the company’s progress. He began by reviewing developments related to Heat’s recently announced, wholly-owned drug discovery and development subsidiary Skunkworx Bio, Inc. Skunkworx features diverse and proprietary libraries of small biologics (“Pocket Biologics”), namely antibody fragments and proteins that have the potential to bind to critical domains of druggable targets and become potential product candidates.
Skunkworx is structured to rapidly and precisely discover and develop new innovative therapeutics. Its biologics library targeting is guided by computational methods and bioinformatics, enhancing efficiency. The subsidiary has already identified modulators for a number of targets and is evaluating them in preclinical in vivo studies to begin by 1Q:22. Skunkworx will develop therapies in both oncology and non-oncology indications as well as in biodefense. Programs started at Skunkworx can be developed at Heat and manufactured by Heat’s other newly announced wholly-owned subsidiary Scorpion Biological Services.

Next, Wolf reviewed the groundbreaking of its new San Antonio Facility announced August 9th. In an effort to augment the company’s internal R&D abilities, Heat is investing in a large molecule bioanalytical research and manufacturing facility under the control of Heat’s wholly owned subsidiary, Scorpion Biological Services. Scorpion will utilize the biomanufacturing/bioanalytic facility for in-house development of immunoassays and biomarkers, while maintaining Good Laboratory Practice (GLP), Good Clinical Practice (GCP), and current Good Manufacturing Practices (cGMP) manufacturing capabilities.
Heat received approval for an estimated $1.0 million in tax abatements from the City of San Antonio and Bexar County. In addition to supporting Heat’s development activities, excess capacity at the facility will be available to support other biopharma companies. Management expects the facility to be self-sustaining through cash flows generated through services rendered to outside companies, all the while supporting Heat’s internal initiatives, including producing product for Heat’s clinical trials. Scorpion is expected to become operational next year and employee headcount is forecasted to reach 50. With cGMP manufacturing in house, Heat will circumvent queues, save costs, improve quality control, and boost flexibility in pursuing programs.
�The Pipeline
The discussion proceeded to review active clinical candidates HS-110, HS-130, PTX-35 and the COVID-19 program.
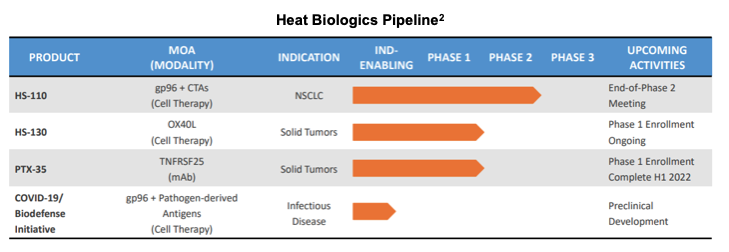
HS-110
Additional data for HS-110 was presented at the 2021 ASCO Annual Meeting held in June, publicized in a June 4th press release. Highlights from the data were a 24.6 month median overall survival (mOS) in previously treated, checkpoint naïve patients with advanced non-small cell lung cancer (NSCLC). The data compared favorably with historical published data of BMS’ nivolumab trial Checkmate 057 which reported mOS of only 12.2 months, single agent, in a similar treatment setting as Phase II HS-110. With these promising data, management now sets its sights on Phase III for the candidate and is actively evaluating registrational pathways with key opinion leaders and others in thorough preparation for strategy talks at the End-of-Phase-II meeting with the FDA, to ensure a smooth meeting and to garner FDA support. Following the meeting, management will be then equipped with details regarding logistics and costs associated with progressing the candidate. Multiple settings are being considered, including patients that are checkpoint naïve as 1L setting, or patients who progress on 1L checkpoints, a 2L setting. Injection Site Reaction, PDL-1 status, tumor mutation burden status are all being considered as biomarkers and management is in conversation with key opinion leaders and oncologists to continue to refine trial design.
HS-130
Heat concluded enrollment in the Phase I trial for HS-130, and is now in the process of evaluating the next steps forward, including enhancements to the platform based on updated information.
Regarding PTX-35, earlier in 2021, Heat announced promising preclinical data at both the AACR Oncology Meeting and 3rd Annual T-reg Directed Therapy Summit. Given the promising data, Heat is expanding (increasing) patient dosing of PTX-35 in patients with solid tumors and expects to update stakeholders with interim data later in 2021. Management is also exploring applications of PTX-35 outside of oncology and is actively pursuing collaborations in various indications.
COVID-19
The COVID-19 program continues. Despite the approval of multiple vaccines, management believes there is opportunity in the emergence of variants, namely the delta variant, that will require combination approaches, and Heat is leveraging expertise into new research to elicit more robust immune response. Clinical trials are very expensive and Heat expected to pursue COVID-19 with government support and will not pursue trials without government support. Heat remains in talks with public support resources. COVID-19 made apparent the general lack of resources and preparedness to respond to new and emerging biosecurity threats, and in response Heat formed its Blackhawk subsidiary focused on emerging biothreats. Blackhawk was originally conceived as a result of research following the pandemic. Management believes that the platform is highly adaptable and can provide versatile and timely responses to protect the US from known and unknown future biological threats.
BioThreat Advisory Board
Heat has filed patents for a novel gp96 approach with biosecurity applications, while strategically keeping much of the research art under wraps. Heat has also established collaborations with leading institutions to further research and plans to provide updates as soon as practical. Heat established a BioThreat Advisory Board, announced August 18th, that is well versed on Blackhawk’s platform, including David Lasseter, Former Deputy Assistant Secretary of Defense for Countering Weapons of Mass Destruction, Former US Representative Jack Kingston, Andrew C. Weber, Former Assistant Secretary of Defense for Nuclear, Chemical & Biological Defense Programs; Dr. Gregory Koblentz, Associate Professor at George Mason University and leading expert on chemical and biological weapons, and Mark Pryor, former US Senator from the State of Arkansas. Together with key appointees on the Biothreat Advisory Board and Heat’s technology and expertise, management sees potential in becoming a leader in the US for biothreat defense.
Financial Matters
CFO Bill Ostrander provided a brief overview of Heat’s financial position. Ostrander reported $122.5 million in cash, equivalents and short term investments as of June 30th, 2021. For the past six months, Heat has been well capitalized and has not utilized its ATM facility. Equity capitalization includes 25.4 million common shares outstanding and just over 747,000 warrants outstanding with an average strike price of approximately $11 per share, generating $8 million if fully exercised.
Summary
In summary, Heat has made progress launching new subsidiaries, breaking ground on its Scorpion San Antonio manufacturing facility, and establishing its Skunkworx subsidiary for the rapid discovery and development of drugs, creating a vertically integrated biologics company with Heat’s technology at the center. Clinical trial results for the year so far have been positive, creating a strong foundation to target next steps, namely Phase III in lead candidate HS-110, with an expected end-of-Phase II meeting with the FDA in the near term.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
________________________
1. Source: Heat Biologics September 2021 Corporate Presentation
2. Source: Heat Biologics September 2021 Corporate Presentation