By David Bautz, PhD
NASDAQ:MNOV
READ THE FULL MNOV RESEARCH REPORT
Business Update
BARDA Contract to Support MN-166 Development for Chlorine Gas-Induced Lung Injury
On March 9, 2021, MediciNova, Inc. (NASDAQ:MNOV) announced a partnership with the Biomedical Advanced Research and Development Authority (BARDA) to test the potential for MN-166 (ibudilast) as a medical countermeasure (MCM) for the treatment of chlorine gas-induced acute respiratory distress syndrome (ARDS) and acute lung injury (ALI). MN-166 will be evaluated in preclinical efficacy models of chlorine gas-induced ALI, and these experiments could help support the use of MN-166 in ARDS/ALI caused by other ailments, including the flu, infections, severe burns, and pancreatitis. Under the FDA animal rule (FDA), development of MCMs do not require human clinical trials to establish efficacy as these would not be ethical or feasible. The FDA can grant approval of a drug for an MCM indication based on well-controlled animal studies, when the results of these studies establish that the drug is reasonably likely to produce clinical benefit in humans.
The release of toxic chemicals, either deliberately through chemical weapons or accidentally through an industrial accident, can result in ALI or ARDS. Chlorine is a widely used industrial chemical (e.g., water purification) that has been previously implicated in both intentional and accidental releases:
• During World War I, Germany launched the first known chemical weapon attack by releasing chlorine gas from 6,000 cylinders against French troops, which caused >1,000 casualties.
• Multiple times in the past decade, the Syrian government of Bashar al-Assad used chlorine as a chemical weapon against its enemies, and a report by the U.S. government in 2020 claimed the regime continues to pursue chemical weapons development.
• In June 2005, a train derailment in South Carolina resulted in the accidental release of 40-60 tons of chlorine gas (Van Sickle et al., 2009). Nine individuals died and >250 were treated for toxic chlorine exposure.
• A mixture of sodium hypochlorite and hydrochloric acid is sometimes used as a cleaning solution. The chlorine gas that is produced can cause airway damage and reactive airways dysfunction syndrome (RADS) (Gorguner et al., 2004).
Chlorine inhalation results in the formation of hydrochloric acid (HCl) and hypochlorous acid (HOCl) as it dissolves into the airway surface liquid. Both of those compounds can result in oxidative injury following the formation of reactive oxygen species, which can result in edema, inflammation, and immediate airway constriction. In addition, chlorine exposure results in the recruitment of inflammatory neutrophils and macrophages (Balakrishna et al., 2014). The inflammatory response is accompanied by increases in various inflammatory markers such as CXCL1, GM-CSF, IL-6, and VEGF.
Current treatment for chlorine inhalation is mostly supportive care. For patients who show airway obstruction, inhaled -2-adrenergic agonists are used (Wang et al., 2004) with early administration of corticosteroids shown to prevent ALI in mouse models (Jonasson et al., 2013). Humidified oxygen is typically administered to all victims, however supplemental oxygen could worsen cardiopulmonary function (Okponyia et al., 2018).
The Department of Homeland Security estimates that a deliberate release of highly concentrated chlorine gas upwind of an urban area with 700,000 individuals would lead to approximately 5% being exposed to a lethal dose of chlorine with approximately 17,500 fatalities and 100,000 hospitalizations (Department of Homeland Security). Chlorine gas is fairly easy to produce, thus the U.S. government is interested in finding MCMs to treat lung injuries caused by chlorine exposure.
The potential market opportunity for MN-166 in chlorine gas-induced ALI is significant as the U.S. government would likely decide to purchase large quantities of the drug for public health emergencies in the homeland (Strategic National Stockpile) and to protect U.S. military personnel abroad.
In addition, drugs approved as MCMs are eligible for a priority review voucher (PRV). A PRV allows the holder of the voucher to receive an expedited six-month review from the FDA for a new drug application (NDA) or biologics license application (BLA) instead of the usual ten-month review. PRVs are fully transferrable and in the past couple of years a number of them have sold for approximately $100 million each.
MN-166 in ARDS Model
MN-166 was previously tested in a lipopolysaccharide (LPS) ARDS mouse model (Yang et al., 2020). While this model induces ARDS through a different mechanism than chlorine gas exposure, a number of the resulting phenotypes are similar between the two models.
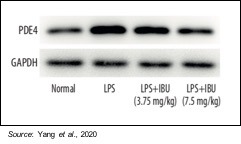
Mice were divided into four groups of 10 each: a control group, a LPS-induced group, and two MN-166 treatment groups (3.75 and 7.5 mg/kg). The following figure shows that PDE4, which MN-166 is an inhibitor of, is increased by LPS stimulation and that treatment with MN-166 decreased this overexpression of PDE4 in lung tissue in ARDS mice.
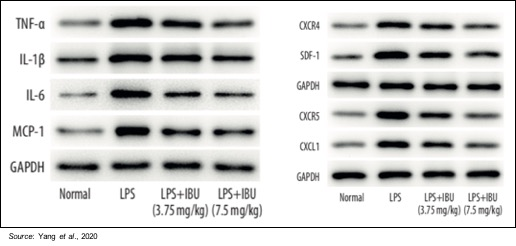
In addition to decreasing the expression of PDE4, treatment with ibudilast also decreases the abnormal overexpression of different inflammatory cytokines, including TNF-a, IL-1b, IL-6, and MCP-1, and inflammatory chemokines, including CXCL1, CXCR4, and CXCR5. As mentioned previously, inflammatory markers such as IL-6 and CXCL1 are also elevated in chlorine gas ARDS models.
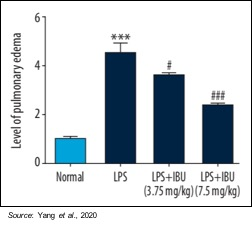
Pulmonary edema was evaluated using the pulmonary edema score to indicate the amount of water accumulation in the lungs after pulmonary damage. Pulmonary edema was significantly reduced by MN-166 treatment (P<0.001). These results suggest that MN-166 may be able to reverse pulmonary edema, which is very important to the recovery of a patient suffering from ARDS, regardless of what induced the condition.
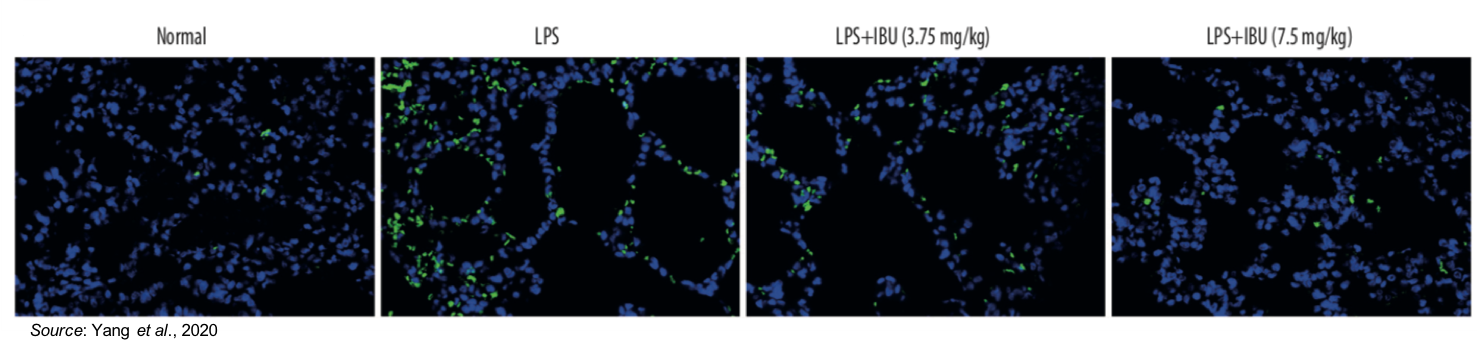
The effect of MN-166 on lung cell apoptosis was also investigated. The following figure shows a TUNEL staining assay (which measures apoptosis; indicated by bright green) in which apoptosis (cell death) is prevalent in the untreated LPS sample. However, the amount of apoptosis is decreased by MN-166 treatment, thus showing the drug’s ability to protect against pulmonary injury.
As ARDS can occur due to a number of different pathogenic insults which all lead to an explosive acute inflammatory response, MN-166’s anti-inflammatory mechanism and its ability to improve the condition in one model (e.g., LPS-induced ARDS) should translate to success in other models (e.g., chlorine gas-induced ARDS). The MN-166 data from the LPS ARDS model was part of MediciNova’s BARDA application, and since the agency deemed it worthy of funding we are excited for its potential and look forward to future updates on the program.
Financial Update
On February 19, 2021, MediciNova filed Form 10-K with financial results for the full year 2020. As expected, the company did not report any revenues. Net loss for 2020 was $13.9 million, or $0.31 per share, compared to a net loss of $12.9 million, or $0.30 per share, for 2019. R&D expenses in 2020 were $7.5 million compared to $6.1 million in 2019. The increase was primarily due to higher clinical trial expenses from the ongoing clinical trial of MN-166 in ALS. G&A expenses in 2020 were $6.7 million compared to $8.0 million in 2019. The decrease was primarily due to decreased non-cash stock-based compensation.
MediciNova exited 2020 with approximately $60 million in cash and cash equivalents and subsequent to the end of the quarter the company received $20 million in gross proceeds in a private placement of stock. We estimate the company has sufficient capital to fund operations at least through the end of 2022, and it’s likely it can finance operations for years beyond then as the company has a successful track record of raising capital. As of February 16, 2021, the company had approximately 48.7 million shares outstanding and when factoring in stock options a fully diluted share count of approximately 56.1 million.
Conclusion
The BARDA grant is an exciting opportunity for MediciNova as MN-166 has previously shown efficacy in a mouse model of LPS-induced ARDS, which we believe bodes well for the drug’s potential as an MCM for chlorine gas-induced ARDS, an indication for which MN-166 can receive FDA approval without doing any human efficacy clinical trials. In addition to ARDS, other MN-166 indications in development include amyotrophic lateral sclerosis (ALS), degenerative cervical myelopathy (DCM), glioblastoma (GBM), chemotherapy induced peripheral neuropathy (CIPN), substance dependence, and progressive multiple sclerosis (MS). The company is also pursuing the development of MN-001 (tipelukast) in NASH and idiopathic pulmonary fibrosis. With approximately $80 million in cash after a private placement, the company is well financed to advance the pipeline and we look forward to updates on these programs as the year advances. Our valuation remains at $26.50.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.