By John Vandermosten, CFA
NASDAQ:NOVN
READ THE FULL NOVN RESEARCH REPORT
B-SIMPLE4 Safety Data
In follow up to the June release of topline results for B-SIMPLE4 and additional detail provided in an update on September 9th, Novan (NASDAQ:NOVN) reported safety data from its pivotal B-SIMPLE4 on September 23rd. The results were in line with previous trials and SB206 was well tolerated. Treatment emergent adverse events (TEAEs) through Week-24 maintained the same favorable profile as SB206’s previous Phase III studies, B-SIMPLE1 and B-SIMPLE2 (see here). The TEAEs reported in greater than 5% of subjects in the SB206 treated groups included pain, erythema, pruritus, exfoliation, and dermatitis at the application site. The majority of these TEAEs were of mild or moderate severity. There were no serious adverse events.
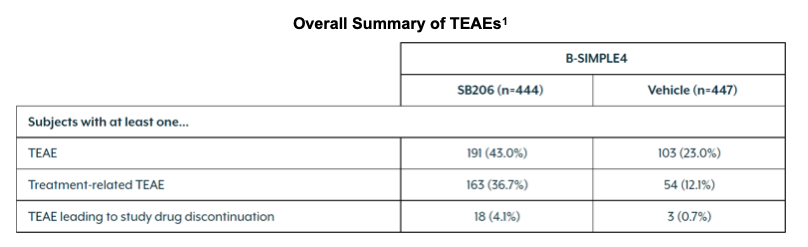
In the SB206 arm, there were 191 (43.0%) subjects with at least one TEAE, of which 163 (36.7%) experienced at least one TEAE that was deemed treatment-related. This compared to 103 (23.0%) and 54 (12.1%) subjects in the vehicle arm, respectively. Some TEAEs led to study drug discontinuation, 18 (4.1%) subjects in the SB206 arm and 3 (0.7%) in the vehicle. Study drug discontinuation in the treatment arm were all due to application site reactions.
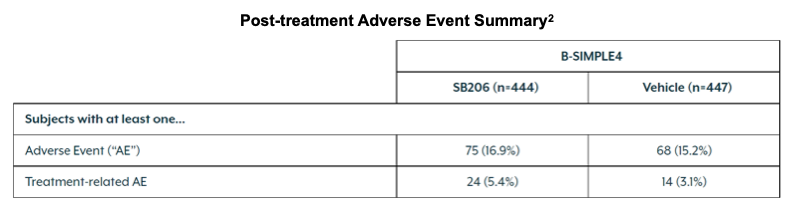
75 (16.9%) and 24 (5.4%) subjects experienced at least one adverse event and treatment-related adverse event, respectively, post treatment in SB206 administered patients. This compared to 68 (15.2%) and 14 (3.1%) subjects in vehicle arm post-treatment.
The incidence of scarring in the SB206 arm was lower than in the vehicle group at 4.7% and 6.3%, respectively. In the Week-24 visit, subjects treated with SB206 also exhibited lower occurrence of scarring compared to vehicle at 2.7% vs 4.0%, respectively. Though a subtle effect and less pronounced than what was observed in the B-SIMPLE1 and 2 safety assessment, the potential added benefit of reduced scarring can be a motivator and of benefit to patients to undergo treatment. Equipped with strong statistically significant endpoints and favorable safety profile, Novan now looks forward to sharing the data with the FDA in a pre-NDA meeting targeted in 1H:22.
September Update
On September 9, 2021, Novan hosted a corporate update conference call and webcast. The call was led by Novan President and CEO, Paula Brown Stafford, who reviewed recent milestones and plans over the next several years. Ms. Stafford detailed Novan’s revenue prospects in the US which are expected to be primarily driven by SB206 for molluscum contagiosum in the near term and SB204 in acne vulgaris and SB019 in COVID-19 in later years. Advancing SB204 allows Novan the possible upside from a relatively small investment, with a large upside with the synergy of commercialization dollars for two dermatology products. Commercialization in the rest of the world is unencumbered from licensing obligations, opening up the field for future partnerships. Cash held on the balance sheet is expected to sustain Novan through NDA submission for SB206, and to support development activities for SB204 and SB019.
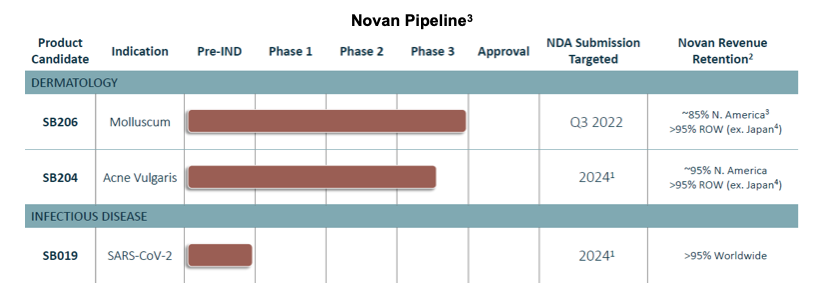
SB206 in Molluscum Contagiosum
CEO Stafford began the update call with a review of SB206’s positive pivotal results in molluscum. Novan’s lead program is expected to produce an NDA submission by 3Q:22. There is an unmet medical need for an at-home, topical solution to treat molluscum contagiosum. Prevalence is estimated at approximately 5-11% of the US population under the age of 16. Novan estimates the addressable market at 6 million individuals, believes payors will reimburse SB206 and the product will appear on formularies.
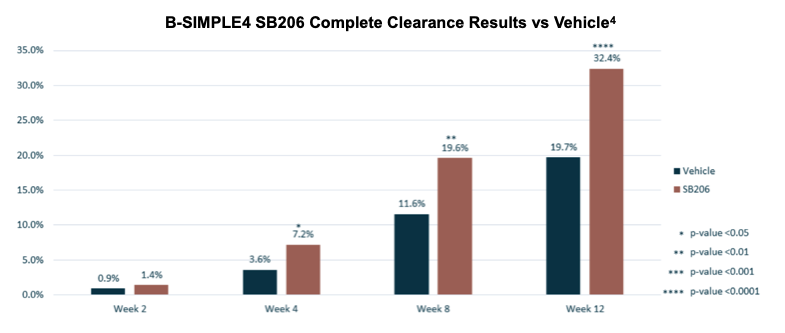
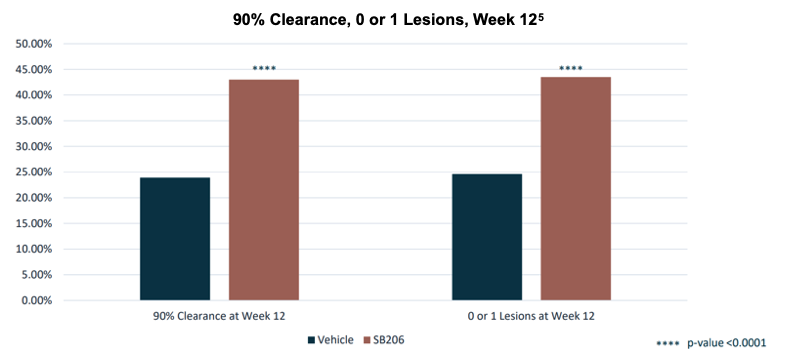
Topline results for B-SIMPLE4 were released in June, achieving a statistically significant primary endpoint of complete clearance of all treatable MC lesions at Week 12, with statistical efficacy observed as early as Week 4. Secondary outcome measures of one-lesion-or-less remaining and 90% clearance, both at Week 12, also showed statistically significant efficacy.
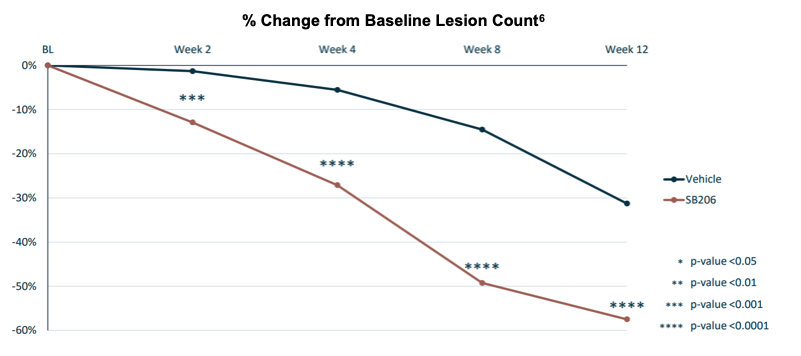
Percent change from baseline, a secondary endpoint, demonstrated statistical significance at every measured time point starting at Week 2.
The CEO provided an update on Novan’s plans for the next few quarters regarding SB206 which is included below:
➢ Pre-commercial preparation;
➢ Trial finalization;
➢ Week 24 readout - September 2021;
➢ Pre-NDA meeting with the FDA - 1H:22;
➢ NDA-enabling stability testing in 1H:22; and
➢ NDA submission - 3Q:22.
Summary
Novan reported favorable safety data, in line with its previous B-SIMPLE1 and B-SIMPLE2 Phase III trials. Equipped with safety data and strongly statistically significant primary endpoints, Novan is now preparing for its pre-NDA meeting with the FDA, expected in 1H:22 and for NDA submission in 2H:22.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
________________________
1. Novan Reports Safety Data from B-SIMPLE4 Pivotal Phase 3 Study of SB206 - Novan, Inc. (gcs-web.com)
2. Novan Reports Safety Data from B-SIMPLE4 Pivotal Phase 3 Study of SB206 - Novan, Inc. (gcs-web.com)
3. Novan Corporate Presentation September 2021
4. Novan Corporate Presentation September 2021
5. Novan Corporate Presentation September 2021
6. Novan Corporate Presentation September 2021