By John Vandermosten, CFA
NYSE:PLX
READ THE FULL PLX RESEARCH REPORT
2020 Financial and Operational Review
Protalix Biotherapeutics, Inc. (NYSE:PLX) announced its 2020 financial and operational results in a March 30, 2021 press release and filing of Form 10-K. The reports were followed by a conference call that morning which discussed recent achievements including results from clinical trials, proceeds from capital raises and upcoming regulatory activity. Key events in 2020 and 2021 year to date include acceptance of a biologics license application (BLA) for PRX-102 and assignment of the April 27, 2021 target action date. Top line and final results from the BRIGHT and BRIDGE studies were announced and the BALANCE study was fully enrolled. Protalix intensified its relationship with SarcoMed, which will investigate PRX-110 in pulmonary sarcoidosis. In the financial sphere, revenues of $62.9 million exceeded our estimates of $53.8 million due to greater research and development revenues from the Chiesi relationship. Loss per share of ($0.22) compared to our forecast of ($0.39).
Financial results for the year ending December 31, 2020, compared to the year ending December 31, 2019:
➢ Revenues were $62.8 million, up 15% from $54.7 million. Sales of Elelyso were up 2% with an improvement in year over year Pfizer sales partially offset by a decline in Brazil sales due to the impact of the pandemic. The R&D services revenues increase of 20% was primarily due to revenue recognized in connection with an updated cost estimation for the completed BRIGHT and BRIDGE studies.
➢ Gross margin (product sales only) rose to 33.0% from 31.3%.
➢ Research and development expenses declined to $38.2 million from $44.6 million, a 14% decline. The decrease was attributable to the completion of the BRIGHT and BRIDGE studies and lower costs from the BALANCE study.
➢ Selling, general and administrative expenses were $11.1 million, up 13% from $9.9 million due to greater share-based compensation, board of directors compensation, partially offset by lower travel, rent and utilities expenditure.
➢ Financial expenses were $9.7 million compared to $8.0 million which relate to interest expense for the convertible notes.
➢ Loss from operations was ($6.5) million compared to ($18.3) million. On a per average share balance, net loss was ($0.22) and ($1.23) respectively.
Cash and equivalents balance on December 31, 2020 was $38.5 million. Following the end of the quarter, additional funds were raised including a gross $40 million from a public equity offering and an additional $9 million from the at-the-money (ATM) program that should bring the cash balance to over $80 million in 1Q:21 after adjusting for financing costs. Cash burn was ($26.8) million, offset by $46.5 million in financing cash flows generated from common stock and warrant issuance, ATM equity offerings and warrant exercise. The strong balance sheet is sufficient to satisfy the $57.9 principal amount outstanding on the convertible notes due in November 2021.
Public Offering
On February 11, 2021, Protalix both proposed a public offering of common stock and announced its pricing. The company ultimately issued 8,749,999 shares at $4.60 per share. Bank of America Securities acted as the book-running manager and Oppenheimer & Co. as the co-manager for the offering. Net proceeds will be used to fund clinical trials for Protalix’ candidates and R&D activities and for working capital for general corporate purposes. Funds will also be available to address the $58 million in convertible notes that mature in November. The completion of the raise was announced February 18, 2021, with gross proceeds totaling approximately $40.25 million and the overallotment exercised in full.
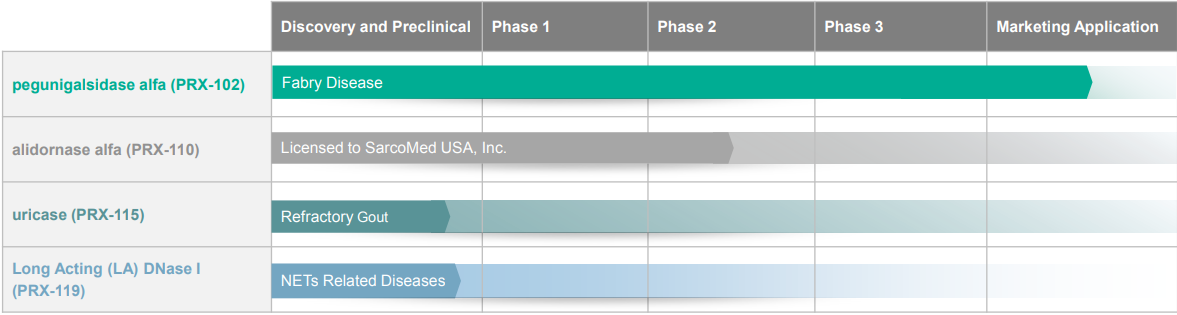
Protalix Clinical Development Pipeline (1)
Exclusive Partnership with SarcoMed USA
Protalix announced on February 11th that it had entered into an exclusive partnership with SarcoMed USA to develop alidornase alfa for the treatment of pulmonary sarcoidosis. This is the culmination of a July 2020 non-binding term sheet between the two companies. SarcoMed USA is a private company that was formed in 2017 to support its lead product candidate, SM001, a recombinant DNase I delivered via inhalation, in pulmonary sarcoidosis. The agreement grants exclusive worldwide license for alidornase alfa (PRX-110), Protalix’ Phase II recombinant DNase I, for use in the treatment of idiopathic pulmonary disorders including, but not limited to, sarcoidosis, pulmonary fibrosis and other related diseases via inhaled delivery.
Under the terms of the agreement, SarcoMed will be responsible for identifying, selecting and conducting clinical research and development of pharmaceutical candidates. In return for the license, Protalix is entitled to upfronts of $3.5 million, subject to conditions, additional payments tied to regulatory and commercial milestones and tiered royalties on product net sales commercialized through the license.
PRX-115
PRX-115 is a plant-cell expressed recombinant PEGylated uricase enzyme in development for refractory gout. This condition affects from 9.2 million to perhaps double that level with more men than women suffering from it. While there are treatments for the disease by way of urate-lowering therapies, many do not respond to it producing an unmet need. Side effects from available medications are severe, and black box warnings for anaphylaxis and strong immunogenic reactions are present. Protalix sees an opportunity with the use of the uricase enzyme, which can convert the uric acid buildup to allantoin, which can be easily excreted from the body. This approach may provide an improved side effect profile and longer term efficacy compared with current treatments.
PRX-119
Protalix introduced PRX-119 in January 2021 as a new enzyme in preclinical work for neutrophil extracellular trap (NET)-related diseases. Excessive formation or ineffective clearance of NETs can cause pathological effects and are present in autoimmune, inflammatory and fibrotic conditions. Preclinical work has shown that DNase treatment may ameliorate NETs toxicity and Protalix anticipates advancing efforts to treat associated acute and chronic conditions with this compound.
PRX-110
Alidornase alfa is recombinant human deoxyribonuclease I (DNase I) expressed via the ProCellEx platform. Admin¬istration is via inhalation for direct application to the lungs. DNase I therapy can act as a mucus thin¬ning agent (mucolytic) to help with clearance from the airways to improve lung function and reduce the chances of infection. Disintegrating inflammatory cells, namely neutrophils, release DNA into the sputum, which polymerizes and is present at high concentrations, contributing to the viscosity of the sputum. DNase I degrades the DNA, thus reducing the viscosity of the mucus.
Clinical Trial Results for PRX-102
PRX–102 is a recombinant α-Galactosidase-A enzyme. Protalix uses its ProCellEx platform to express the enzyme and then chemically modifies it via surface pegylation. Protein sub-units are co¬valently bound via chemical cross-linking using short PEG moieties, resulting in a molecule with unique pharmaco¬kinetic parameters. In clinical studies, PRX–102 has demonstrated a circulatory half-life of approximately 80 hours. Due to the chronic na¬ture of Fabry, patients must receive IV infusion of enzyme replacement therapy every two weeks, which is a signifi¬cant burden. PRX-102, with its extended half-life, aims not only to be more effective, but also reduce the frequency of doctors’ visits by Fabry patients.
Three Phase III studies were launched to support regulatory approval of PRX-102 around the globe, designated BRIDGE, BALANCE and BRIGHT. After a release of topline results in May 2020, the BRIDGE trial provided final results on December 30, reiterating its findings of a substantial improvement in renal function. See our March 31 report for details on trial outcomes.
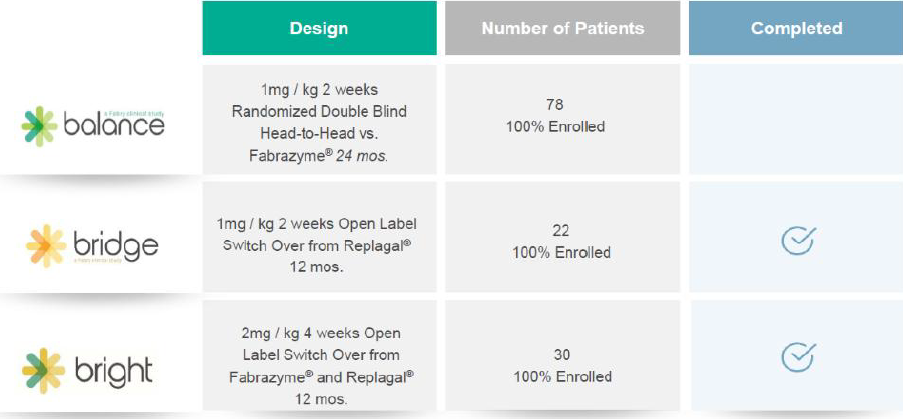
PRX-102 Phase III Trial Comparison (5)
Summary
Since our recent initiation on Protalix, the company has provided several updates including closing almost $50 million in gross proceeds year to date, expanding a partnership with SarcoMed USA, announcing of final results from BRIDGE and providing topline data from BRIGHT. We also anticipate positive news from the FDA regarding approval of PRX-102 in the next month. Assuming FDA approval, sales of the enzyme should be underway by 2H:20. Below we summarize the key elements of our thesis:
➢ PRX-102 target action date of April 27, 2021, subsequent approval and commercialization
➢ Potential for superiority vs market leader Fabrazyme
➢ Existing sales and royalty revenues from taliglucerase alfa
➢ Orphan indication for PRX-102
➢ Partnership with Chiesi for global commercialization of PRX-102 in Fabry Disease
➢ Rights to milestones and royalties
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
________________________
1. Protalix Corporate Presentation March 2021
2. Singh, G. et al. Gout and Hyperuricaemia in the USA: Prevalence and Trends. Rheumatology. 2019;58(12):2177-2180.
3. Dehlin, M. et al. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol
4. Pressler T. (2008). Review of recombinant human deoxyribonuclease (rhDNase) in the management of patients with cystic fibrosis. Biologics: targets & therapy, 2(4), 611–617. https://doi.org/10.2147/btt.s3052
5. Source: Protalix 2020 Form 10-K