By David Bautz, PhD
NASDAQ:TNXP
READ THE FULL TNXP RESEARCH REPORT
Business Update
TNX-1800 Shows Efficacy in Non-Human Primates
On March 17, 2021, Tonix Pharmaceutical Holdings Corp. (NASDAQ:TNXP) announced positive efficacy results for TNX-1800, the company’s lead COVID-19 vaccine, in non-human primates. The study was designed to compare TNX-1800 (modified horsepox virus encoding SARS-CoV-2 spike protein) to TNX-801 (horsepox virus) at two different doses with a control group receiving placebo. Four animals were included for each of the five groups.
Forty-one days following vaccination (or placebo), each animal was administered SARS-CoV-2 by intra-tracheal (1 x 106 TCID50) and intra-nasal (1 x 106 TCID50) administration. TCID50 is a method for quantifying virus particles and represents a dilution that causes 50% of cells to display cytopathic effects. Six days after viral challenge, upper airway virus was examined through oropharyngeal swab and lower airway virus by tracheal lavage using qRT-PCR to quantitate the number of genome copies of SARS-CoV-2 present. The results showed that no samples (0/8) from animals vaccinated with TNX-1800 showed infection (defined as > 1,000 genome copies of SARS-CoV-2) in either the upper or lower airways. All animals vaccinated with TNX-801 (8/8) or placebo (4/4) showed infection in either the upper or lower airways.
Tonix had previously disclosed immunogenicity data for the same animals in November 2020. On Day 14 after vaccination, all (8/8) TNX-1800-vaccinated animals had anti-CoV-2 neutralizing antibodies (≥1:40 titer) while none of the TNX-801 (0/8) or placebo-vaccinated (0/4) developed anti-CoV-2 neutralizing antibodies (≤1:10 titer). Six days after challenge with SARS-CoV-2, TNX-1800-vaccinated animals showed neutralizing antibody titers (≥1:1280 titer), which was similar between the low and high dose TNX-1800 groups (1 x 106 PFU and 3 x 106 PFU, respectively). In addition, all vaccinated animals developed a ‘take’, which is a small lesion at the site of the immunization of a live, replicating virus vaccine that occurs approximately one week following dosing. The ‘take’ is simple biomarker of a strong T cell immune response and is important as it is costly to measure the T cell response to a vaccine through in vitro studies.
These results show that TNX-1800 induces a protective immune response to SARS-CoV-2 following a single immunization in non-human primates. In addition, it confirms that the ‘take’ in those vaccinated with TNX-1800 is a biomarker of protection of both upper and lower airways from SARS-CoV-2 challenge. The next step will be filing of an IND such that a Phase 1 trial in healthy volunteers can initiate in the second half of 2021, once cGMP vaccine material is available.
Update on Oxytocin Platform
Tonix has established its oxytocin programs over the past year through the following acquisitions:
In June 2020, Tonix announced the acquisition of TNX-1900, a proprietary, patented enhanced formulation of intranasally administered oxytocin for the treatment of chronic migraine and craniofacial pain.
In December 2020, Tonix announced the acquisition of an exclusive license for using intranasal oxytocin to treat insulin resistance and related syndromes, including obesity.
In February 2021, Tonix announced the licensing of technology for the use of intranasal oxytocin in the treatment of Prader-Willi syndrome, a rare genetic disorder that results in uncontrolled appetite and obesity in childhood and adulthood.
Migraine
A migraine is a headache that can cause severe pain, can last up to 72 hours, has a pulsing quality, and may be associated with nausea, vomiting, phonophobia, or photophobia. Approximately one billion individuals in the world suffer from migraine headaches (GBD 2016 Headache Collaborators). In the U.S., migraines affect approximately 72 million individuals (Gooch et al., 2017). Chronic migraines, which are defined as having a headache at least 15 times a month for three months, are less common (1-5% of migraine sufferers). While not a fatal condition, migraines still contribute to a significant decrease in quality of life and the total estimated cost of all migraine headaches is approximately $78 billion per year (Gooch et al., 2017).
Migraine treatments are classified as abortive (intended to stop a migraine once it begins) or prophylactic (decrease the frequency and/or severity of migraines). Abortive treatments include triptans and ditans (which specifically target serotonin receptors), over the counter medications (that typically include some combination of a nonsteroidal anti-inflammatory drug (NSAID), acetaminophen, and/or caffeine), ergots, and calcitonin gene-related peptide (CGRP) antagonists.
Antibodies that target the interaction of CGRP with its receptor represent the newest class of prophylactic treatments and include Aimovig (erenumab), which targets the CGRP receptor, and Ajovy (fremanezumab), Emgality (galcanezumab), and Vyepti (eptinezumab), which each target CGRP. These drugs generated approximately $1 billion in sales in 2020, and are estimated to generate 2026 sales of $4 billion (EvaluatePharma). A summary of their results in Phase 3 clinical trials is shown in the following table.
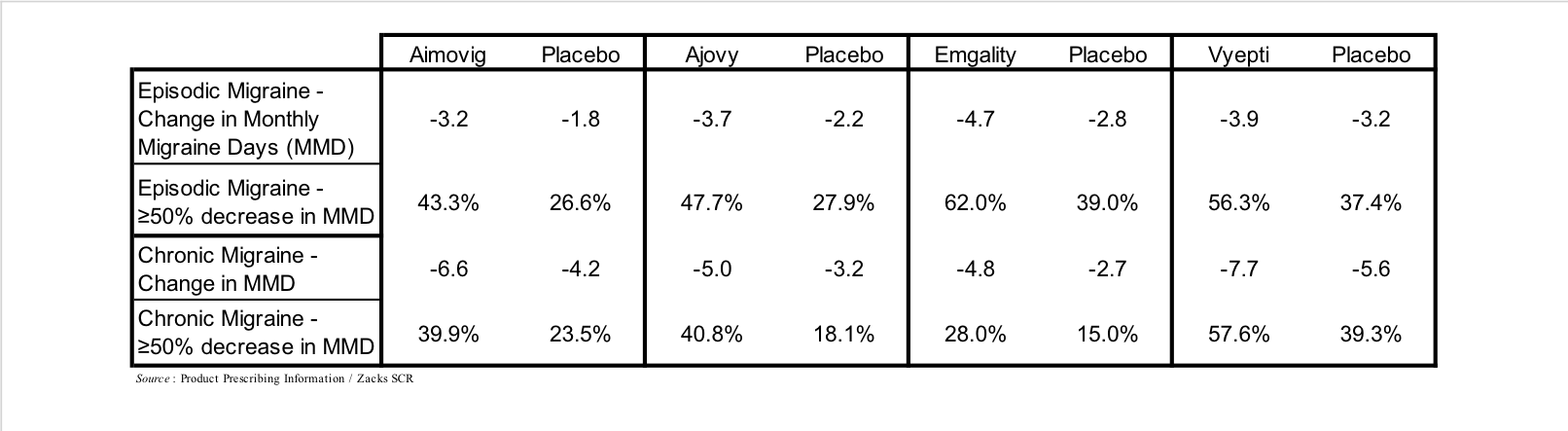
Each of the CGRP-directed antibody treatments are administered as subcutaneous injections or infusions on a monthly or once every three months schedule. Side effects associated with CGRP antibodies are generally mild or moderate, with redness and/or pain at injection site the most common, although due to a number of core systems that CGRP is involved in there are a number of theoretical long-term adverse effects that may arise once the data (≥10 years) is available, including CGRP’s role in hypertension, wound healing, and insulin levels (Robbins, 2020).
Oxytocin in Migraine
While the exact cause of migraines remains to be elucidated, it appears to be associated with the hypothalamus and its connections with the spinal trigeminal nuclei and sensory trigeminovascular system, thus neuromodulators that target the trigeminal pathway could prove effective in migraines.
Oxytocin is a nine amino acid peptide hormone that is involved in a wide array of biological processes, including learning and memory, anxiety, addiction, feeding behavior, maternal behavior, and processing of social information (Cilz et al., 2019). A review of the available literature showed that in 29/33 animal studies oxytocin increased “pain” tolerance (Rash et al., 2014), which suggests it may act as an analgesic. Circumstantial evidence shows that oxytocin may be an effective migraine treatment based on the fact that increased oxytocin levels in pregnancy and during breastfeeding correlates with decreased migraine occurrence (Hoshiyama et al., 2012) and women who suffer from migraines report that sex (which results in a surge in oxytocin levels) provides at least temporary relief from the condition (Evans et al., 2001).
While the potential for oxytocin as a treatment for migraine headaches appears promising, getting oxytocin to the trigeminal system is challenging due to the fact that: 1) oxytocin is a small peptide that is broken down almost immediately in the gastrointestinal system; and 2) its half-life in the blood stream is very short (3-5 min) with only limited ability to cross the blood-brain barrier, thus eliminating the availability of oral or parenteral administration. But delivered intranasally, oxytocin permeates the nasal mucosa and is transported along the trigeminal nerve pathway to the trigeminal ganglion where it can inhibit release along trigeminal neurons that synapse within the lining of the dura mater and inhibit CGRP release. Additionally, the most direct way to bypass the blood brain barrier and gain access to the CNS could be nasal administration along olfactory and trigeminal pathways to the brain (Dhuria et al., 2010).
Multiple preclinical studies have examined the potential for oxytocin as a migraine treatment. Oxytocin receptors are present in rat trigeminal neurons, and the vast majority of neurons that express oxytocin receptors also express CGRP (Tzabazis et al., 2016). Intranasal administration of radiolabeled oxytocin in rats results in a very high concentration of oxytocin in all three branches of the trigeminal nerve and trigeminal ganglion (Tzabazis et al., 2017). Administration of nitroglycerin triggers migraine headaches in patients (Sances et al., 2004), thus intraperitoneal injection of nitroglycerin is utilized in a rat model of migraine (Ma et al., 2008). This model results in expression of C-fos in multiple trigeminal neurons (a marker for trigeminal nerve activation), however pre-treatment with intranasal oxytocin markedly reduces the expression of C-fos, thus pointing to a potential role of oxytocin in preventing pain transmission.
A Phase 2 clinical trial of intranasal oxytocin was previously conducted by Trigemina, Inc., from which Tonix acquired TNX-1900 in June 2020. This was a double blind, placebo controlled trial in 218 mostly female migraine sufferers (143 on oxytocin; 75 on placebo) and was conducted in Chile, Australia, and New Zealand (NCT01839149). The trial consisted of a 28-day “run-in” period to establish a baseline of migraine days followed by 56 days of “as needed” dosing with either intranasal oxytocin or placebo. Results showed that while intranasal oxytocin was well tolerated, the study did not meet the primary endpoint of a reduction in migraine headache days from baseline. This was mostly due to an extremely high placebo response rate at the clinical sites in Chile, which was 74%, while subjects from New Zealand and Australia experienced a normal placebo response and showed a statistically significant difference between active and placebo groups (Tzabazis et al., 2017).
Based on results of preclinical studies demonstrating enhanced analgesic activity of oxytocin mediated by the oxytocin receptor in the presence of magnesium, Trigemina developed a proprietary, potentiated formulation of oxytocin containing magnesium. Tonix acquired the intellectual property for this formulation and plans to advance it first in the study of chronic migraine.
To follow up on the results of the prior Phase 2 trial, we anticipate Tonix conducting a similarly designed Phase 2 trial of TNX-1900, which is the potentiated formulation of intranasal oxytocin, for the prophylactic treatment of chronic migraine. The company is expected to file an IND for TNX-1900 in the second quarter of 2021 such that a trial can initiate in the third quarter of 2021. We expect the trial to be similarly designed to the previous Phase 2 trial with a 28-day baseline period followed by 84 days of dosing.
Financial Update
On March 15, 2021, Tonix announced financial results for the fourth quarter and full year 2020. As expected, the company did not report any revenues for the fourth quarter or full year 2020. Net loss for the fourth quarter of 2020 was $17.0 million, or $0.10 per share, compared to a net loss of $11.2 million, or $2.86 per share, for the fourth quarter of 2019. The weighted average common shares outstanding for the fourth quarter of 2020 were approximately 163.9 million compared to approximately 3.9 million for the fourth quarter of 2019.
R&D expenses for the fourth quarter of 2020 were $12.1 million, compared to $5.7 million for the fourth quarter of 2019. The increase was primarily due to two ongoing Phase 3 clinical trials for TNX-102 SL for fibromyalgia along with the development of TNX-1800, which was not in development in 2019. G&A expenses for the fourth quarter of 2020 were $4.9 million, compared to $3.0 million for the fourth quarter of 2019. The increase was primarily due to an increase in financial reporting expenses, patent expenses, and an increased headcount.
For 2020, net loss available to stockholders was $52.2 million, or $0.55 per share, compared to a net loss of $31.1 million, or $19.33 per share, for 2019. The weighted average common shares outstanding for full year 2020 was approximately 94.6 million, compared to approximately 1.7 million for full year 2019. R&D expenses in 2020 were $36.2 million, compared to $18.2 million for 2019. The increase was primarily due to the timing of development milestones for the Phase 3 fibromyalgia trials in 2020, increased activities for TNX-1800, and the Trigemina asset acquisition. G&A expenses for 2020 were $14.4 million, compared to $10.6 million for 2019. The increase is primarily due to increased legal fees, patent prosecution, and increased headcount.
As of December 31, 2020, Tonix had approximately $77.1 million in cash and cash equivalents. Subsequent to the end of the year, the company strengthened its balance sheet through multiple financing transactions. In January 2021, Tonix raised gross proceeds of approximately $40M from the sale of 50 million shares of common stock at $0.80 per share. In February 2021, Tonix raised gross proceeds of approximately $70M from the sale of approximately 58 million shares at a price of $1.20 per share. We estimate the company currently has approximately $170 million in cash and cash equivalents. As of March 15, 2021, Tonix had approximately 323.9 million shares outstanding.
Conclusion
The efficacy results presented for TNX-1800 in non-human primates are very encouraging and support its advancement into human trials. We anticipate a Phase 1 clinical trial in healthy volunteers initiating in the second half of 2021. Even with multiple vaccines being utilized under an Emergency Use Authorization (EUA), we still do not know how long those vaccines are efficacious or if they offer protection against emerging variants. We believe a vaccine with a T cell predominant response like TNX-1800 may be effective against SARS-CoV-2 variants with the potential for longer immunological protection.
Upcoming milestones include the initiation of a Phase 2 open label safety study of TNX-1300 for cocaine intoxication, the submission of an IND for TNX-2100 for SARS-CoV-2 skin test, and the submission of an IND for TNX-1900 for the treatment of migraine in the second quarter of 2021. In addition, we anticipate the initiation of the Phase 2 clinical trial of TNX-1900 for the treatment of chronic migraine and the results of the interim analysis of TNX-102 SL in the Phase 3 RALLY study in fibromyalgia in the third quarter of 2021, with topline results for the RALLY study in the fourth quarter of 2021. With no changes to the model our valuation remains at $3.75.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.