By David Bautz, PhD
NASDAQ:MDNA
READ THE FULL MDNA RESEARCH REPORT
Business Update
Confirmed Partial Response in ABILITY Trial
On September 28, 2022, Medicenna Therapeutics Corp. (NASDAQ:MDNA) announced new clinical data from the ongoing Phase 1/2 ABILITY Study (A Beta-only IL-2 ImmunoTherapY Study) of MDNA11 in patients with advanced solid tumors (NCT05086692). The new data includes a confirmed partial response (PR) in a patient with metastatic pancreatic ductal adenocarcinoma (PDAC) that had failed prior chemotherapy and checkpoint inhibitor therapies. The confirmatory scan for that patient showed that they continue to show tumor reduction when compared to prior scans. Overall, of the 14 evaluable patients, five have achieved tumor control (PR or stable disease [SD]) with MDNA11 monotherapy. The five patients that have achieved tumor control are listed below, with a summary of all patients treated thus far in the following chart:
1. Fourth-line (4L) PDAC Stage IV (Dose Level 4 @ 60 μg/kg following two divided doses of 30 μg/kg); confirmed PR
2. 3L non-clear cell renal cell carcinoma (Dose level 4 @ 60 μg/kg); SD
3. 4L sarcoma (Dose level 3 @ 30 μg/kg); SD
4. 3L melanoma (initial dose level 1 @ 10 μg/kg; escalated to dose level 3 @ 30 μg/kg and dose level 4 @ 60 μg/kg); SD
5. 3L sarcoma (initial dose level 1 @ 10 μg/kg); SD
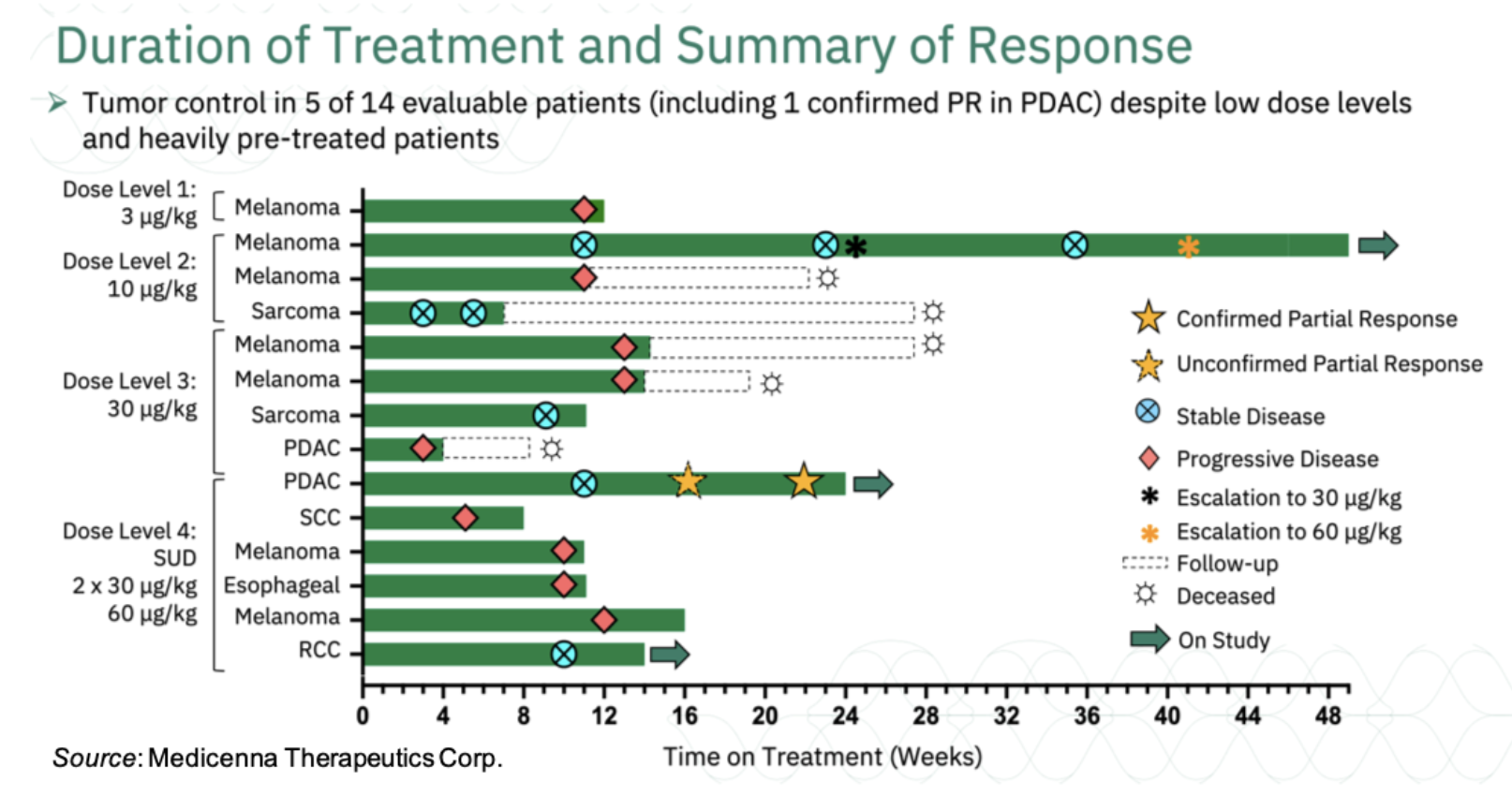
Importantly, MDNA11 continues to demonstrate a favorable tolerability profile as a monotherapy. To date, there have been no reports of dose-limiting toxicities, dose interruptions, dose de-escalations, or treatment discontinuations due to safety issues.
We anticipate a detailed presentation on MDNA11’s safety, pharmacokinetic, and pharmacodynamic profiles at a major medical conference in the fourth quarter of calendar 2022.
On September 13, 2022, Medicenna announced a clinical collaboration and supply agreement with Merck to evaluate MDNA11 in combination with Keytruda® (pembrolizumab) in the ABILITY study. Under terms of the agreement, Medicenna will continue to sponsor the study and Merck will supply pembrolizumab. A Joint Development Committee has been established by the two companies to advance the combination portion of the study. The combination portion of the trial is likely to start in mid-2023.
Preclinical Data Presented at Cytokines 2022
On September 22, 2022, Medicenna announced the presentation of preclinical data from two programs demonstrating the anti-tumor activity of MDNA223 (anti-PD1-IL-2 BiSKIT) and MDNA413 (long-acting IL-4/IL-13 super-antagonist). Both presentations were featured in posters at the 10th Annual Meeting of the International Cytokine & Interferon Society. A copy of the MDNA223 presentation can be found here. A copy of the MDNA413 poster can be found here.
A Next Generation Bifunctional Superkine for Immunotherapy (BiSKIT) Encompassing the Combined Therapeutic Potency of IL-2 Super-Agonist and Anti-PD1
MDNA223 consists of an anti-PD1 antibody covalently linked to an IL-2 super-agonist (MDNA109FEAA). The compound facilitates cis-binding to IL-2R and PD1 on the same cell, which potentiates the synergy between IL-2 agonism (to stimulate CD8+ T cell activation) and PD1/PDL1 blockade (to prevent CD8+ T cell exhaustion).
The following image shows that MDNA223m (the mouse surrogate of MDNA223) induces a prolonged pharmacodynamic (PD) response that extends beyond the duration of the serum exposure. Following a single dose of MDNA223m (2 mg/kg), the pharmacokinetic (PK) profile shows a decline in the serum concentration of MDNA223m but with an increase in CD8+ T cell expansion through Day 7. This increase in CD8+ T cells is also not accompanied by any increase in Treg cells.
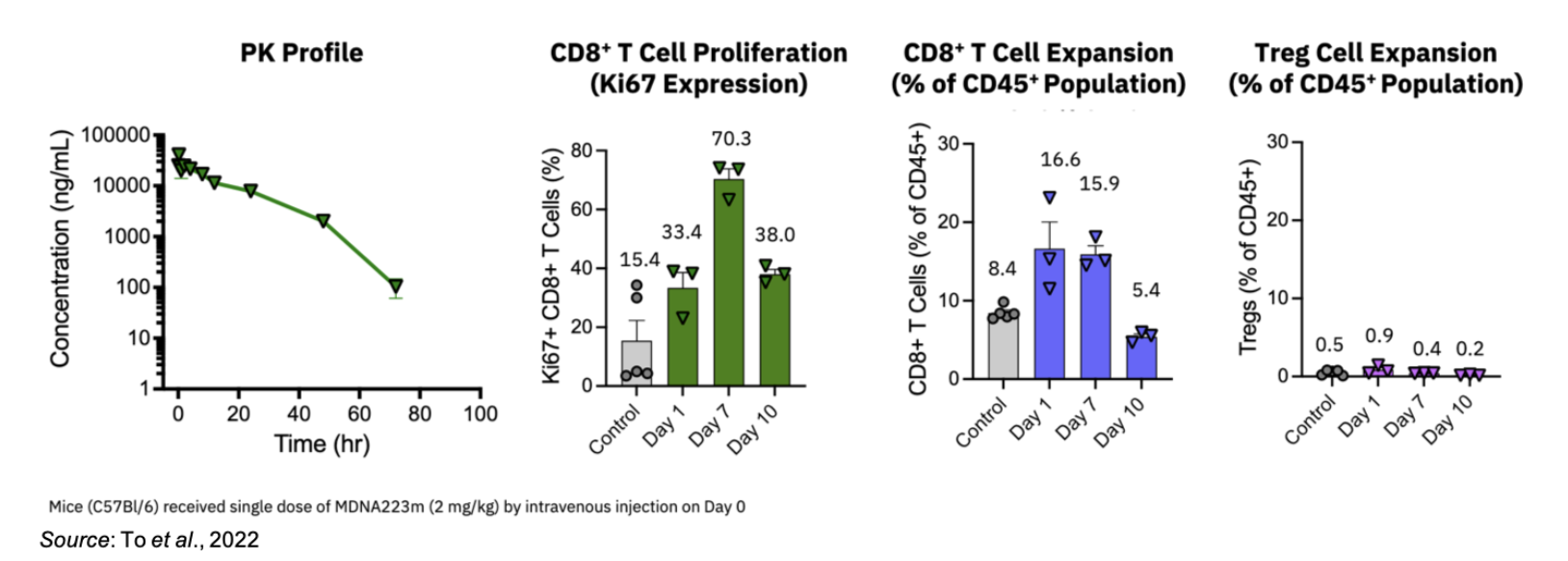
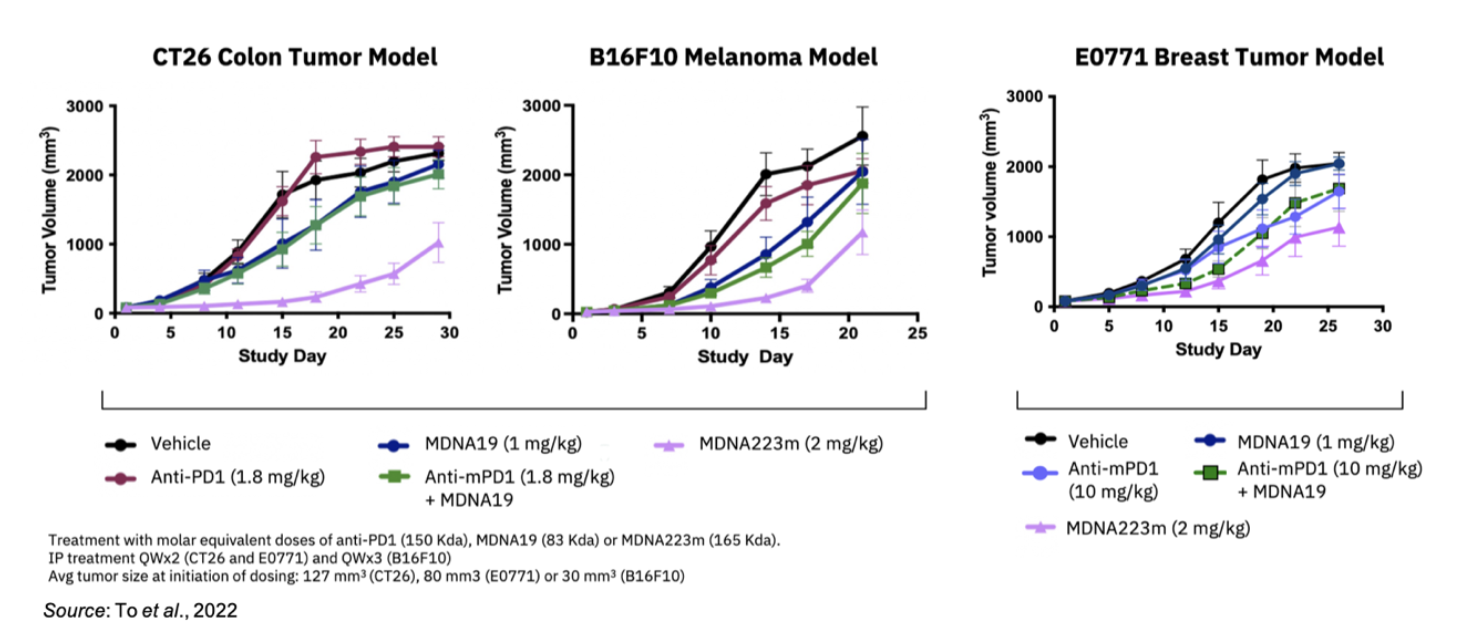
In addition to the extended PD effect, MDNA223m exhibited superior efficacy in three separate mouse tumor models (colon, skin, breast) compared to co-administration of an anti-PD1 antibody and MDNA109FEAA. This shows that the synergy resulting from MDNA223 concurrently targeting PD1 and IL-2 receptor on the same cell leads to an enhanced response compared to co-administration of anti-PD1 and IL-2 therapies.
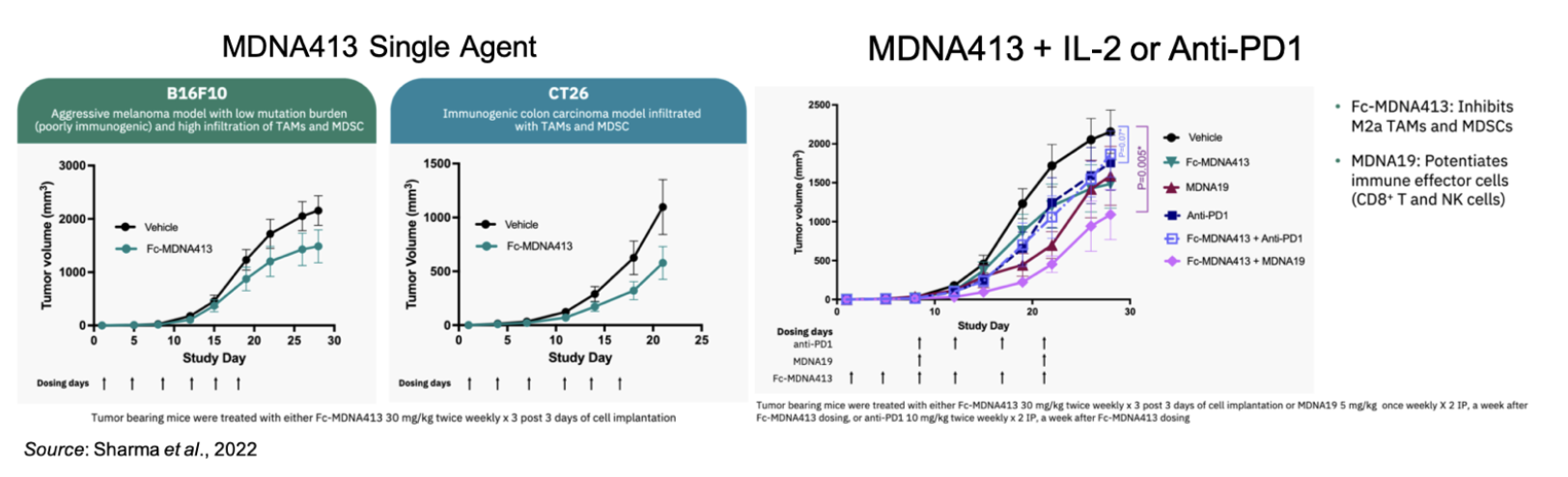
Fc-MDNA413 is a Novel Long-Acting IL-4/IL-13 Super-Antagonist that Suppresses M2a TAM Skewing and In Vivo Tumor Growth Including Synergy with an IL-2 Super-Agonist
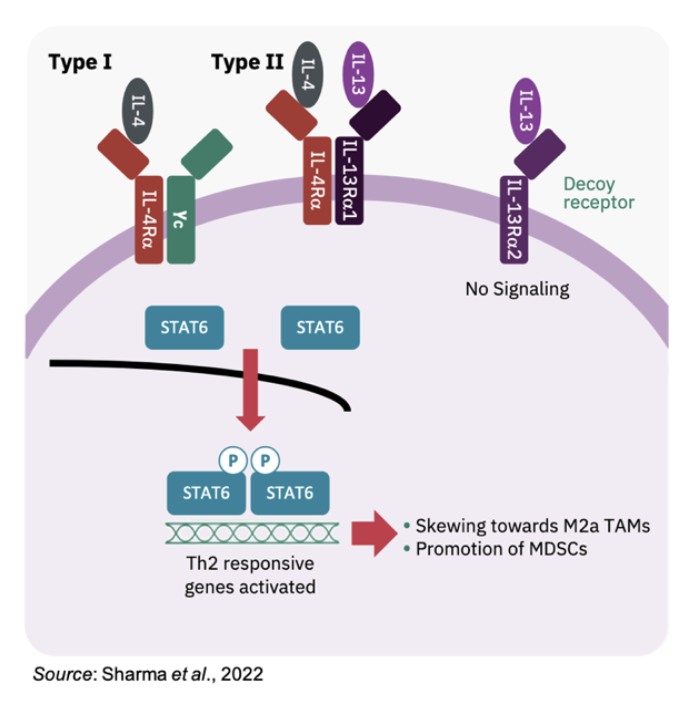
Fc-MDNA413 is an IL-13 super-antagonist that binds to the Type II IL-4 receptor (made up of IL-4α/IL-13Rα1) that is expressed on tumor associated macrophages (TAMs) and myeloid derived suppressor cells (MDSCs). An overview of IL-4/IL-13 signaling is shown below. Binding of native IL-13 to the Type II receptor activates the Stat6 signaling pathway that ultimately promotes TAMs and MDSCs. Both of these cell types limit immune effector cells and promote an ‘immunologically cold’ tumor microenvironment (Bhattacharjee et al., 2013).
In vitro analysis showed that MDNA413 is 300-times more selective for IL13Rα1 over IL-13Rα2 compared to a fusion protein consisting of an Fc domain linked to wild type IL-13. In addition, mutations in MDNA413 selectively blocks signaling via the type 2 IL4R. This enhanced binding capability and blockade of signaling resulted in an inhibition of IL-4/IL-13 mediated functions as measured by pSTAT6 signaling, TF-1 cell proliferation, and M2a polarization of macrophages. In two murine tumor models (melanoma and colon cancer), MDNA413 showed single agent activity that resulted in tumor growth inhibition and it also synergized with an IL-2 agonist, showing the advantages of targeting suppressive and effector immune cells within a “cold” tumor microenvironment.
Conclusion
The confirmation of the PR in the ABILITY trial, along with four additional patients that have achieved tumor control, is very exciting and provides compelling evidence of MDNA11’s single-agent activity. We look forward to an update on the trial at a major scientific conference in the fourth quarter of 2022 and we anticipate additional efficacy updates in the first quarter of 2023. The recently announced clinical collaboration with Merck is an important cost-saving measure as it will allow Merck to supply pembrolizumab instead of Medicenna having to procure it on its own. With no changes to our model our valuation remains at $7 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.