By David Bautz, PhD
NASDAQ:TNXP
READ THE FULL TNXP RESEARCH REPORT
Business Update
TNX-1500 to Enter Phase 1 Clinical Trial in 2H22
Tonix Pharmaceuticals Holding Corp. (NASDAQ:TNXP) recently highlighted new preclinical data for TNX-1500 showing long-term rejection free graft survival in heart and kidney allografts in non-human primates (NHPs). The studies were conducted at Massachusetts General Hospital/Harvard Medical School. TNX-1500 is a third-generation anti-CD40 ligand (CD40L) monoclonal antibody (mAb) that is being developed for the prevention of allograft rejection, xenotransplantation, and the treatment of autoimmune disease.
The CD40/CD40L signaling pathway is involved in the activation of both the innate and adaptive immune response. CD40 is predominantly expressed on antigen presenting cells (APCs) and delivers intracellular activating signals. CD40L, which does not contain any signaling capacity, is found on multiple cell types, including T cells, B cells, natural killer (NK) cells, macrophages, and platelets (Schönbeck et al., 2001). The CD40/CD40L pathway is essential for humoral immune responses to T cell-dependent antigens (Lederman et al., 1992), the production of proinflammatory cytokines (Cella et al., 1996), and generating effective cytotoxic T cell responses (Liu et al., 2013).
For the heart allograft study in NHPs, TNX-1500 was dosed at 30 mg/kg twice weekly on days 0, 3, 7, and 14 followed by weekly dosing of 20 mg/kg from days 21 to 175. Results showed that in 4/5 heart transplants, there was no acute cellular injury (as judged by H&E staining) or chronic antibody injury (as judged by CD4 immunohistochemistry). The animals are currently being observed following cessation of therapy, but thus far there is prolonged organ acceptance. These results are similar to what was seen with hu5c8, a mouse/human IgGk1 chimeric anti-CD40L mAb, however treatment with hu5c8 caused thrombosis in some animals, which was not seen with TNX-1500.
For the kidney allo-transplantation study in NHPs, TNX-1500 was dosed at 20 mg/kg on days 0, 2, 7 and then weekly until day 180. The following chart shows that of the six recipients, no rejection was observed in five of them. This compares to treatment with conventional immunosuppressive therapy, in which 10 of 20 recipients showed organ rejection by day 120. The four recipients that did not receive any therapy all rejected the organ within a few days.
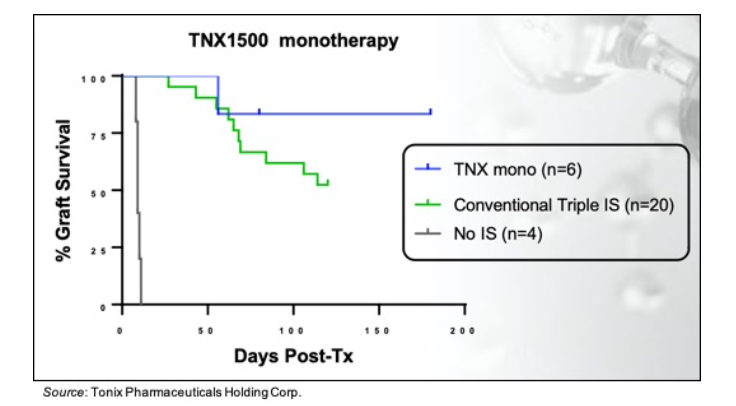
Another strategy being employed to facilitate long-term tolerance induction is the use of a combined kidney and bone marrow transplant (CKBMT; Kawai et al., 2008, Kawai et al.,2014). The end goal of this procedure is to have long-lasting, durable tolerance without the need for immunosuppressive therapy. The first step in the procedure is to severely deplete the recipients mature T cells. New protocols for T cell depletion are being developed that utilize anti-CD40L and/or anti-CTLA mAbs. In an NHP CKBMT trial, an NHP recipient received a conditioning regiment consisting of low dose total body irradiation, thymic irradiation, venetoclax, and thymoglobulin followed by a CKBMT. Four doses of TNX-1500 were administered over seven days and immunosuppressive treatment continued until day 28. At one year, the kidney biopsy showed no signs of rejection, meaning the recipient achieved long-term immunosuppression-free survival.
In addition to its potential utility in preventing allograft rejection, TNX-1500 may also be utilized to prevent rejection in xenograft transplants. Given the shortage of human donor organs currently available, xenografts utilizing organs procured from genetically modified pigs may become a viable alternative. Multiple changes to the pig’s genome aid in preventing tissue rejection, however following the transplant it is still necessary for the patient to be administered costimulation pathway blockers to prevent cell-mediated immune rejection. Previous work has shown that blockade of the CD40-CD40L interaction is associated with the longest pig-to-primate xenograft survivals (Samy et al., 2017).
Lastly, TNX-1500 may be utilized as a treatment for autoimmune diseases such as rheumatoid arthritis, psoriasis, multiple sclerosis, systemic lupus erythematosus (SLE), and Crohn’s disease. In mice, genetic knockout of CD40L results in protection from multiple experimental autoimmune diseases, including experimental allergic encephalomyelitis (Grewal et al., 1996). In addition, CD40L expression is increased on a number of cell subsets in patients with systemic lupus erythematosus (Desai-Mehta et al., 1996) and on circulating T cells from patients with rheumatoid arthritis and psoriatic arthritis (Daoussis et al., 2006), thus supporting its role in autoimmune disease. Development of first-generation anti-CD40L mAbs had to be halted after multiple reports of thromboembolic complications (Kawai et al., 2000). This risk is thought to result from the IgG constant region interacting with FcγRIIA (Robles-Carrillo et al., 2010). Second- and third-generation anti-CD40L mAbs have been engineered to reduce binding to FcγRIIA, and while second-generation molecules have exhibited decreased efficacy, TNX-1500 is designed to preserve FcRn function and deliver efficacy without an increased safety risk.
Tonix will be conducting a pre-IND meeting with the FDA such that a Phase 1 clinical trial can be initiated in the second half of 2022. The first indication for TNX-1500 will be kidney allotransplantation, with the goal of replacing nephrotoxic calcineurin inhibitors. Additional indications will include heart or kidney xenotransplantation, the treatment of the rare neurodegenerative condition amyotrophic lateral sclerosis (ALS), and autoimmune diseases. The company has filed composition of matter patents for TNX-1500 and manufacturing of the antibody is in progress in preparation for the first clinical trial.
Update on TNX-102 SL for Fibromyalgia
On March 21, 2022, Tonix announced that, as expected based on a previously reported pre-specified interim analysis, TNX-102 SL did not achieve the primary endpoint of reducing fibromyalgia (FM) daily pain at Week 14 in the Phase 3 RALLY trial. The RALLY study was a 14-week randomized, double blind, placebo controlled trial of TNX-102 SL 5.6 mg in which 514 subjects with FM were randomized 1:1 to receive either TNX-102 SL 2.8 mg or placebo for the first two weeks followed by TNX-102 SL 5.6 mg (2 x 2.8 mg tablets) or two placebo tablets for the remaining 12 weeks.
In December 2020, Tonix announced positive topline results for the Phase 3 RELIEF study of TNX-102 SL 5.6 mg (primary endpoint, P=0.010). The disparate outcomes between the RELIEF study and RALLY study are hard to reconcile, however a look at the results of the RALLY study may offer some insight into why it was unsuccessful.
There was a 79% and 77% increase in the number of adverse event-related participant discontinuations in the drug treatment group and placebo group, respectively, in the RALLY study compared to the RELIEF study, as shown in the following table.

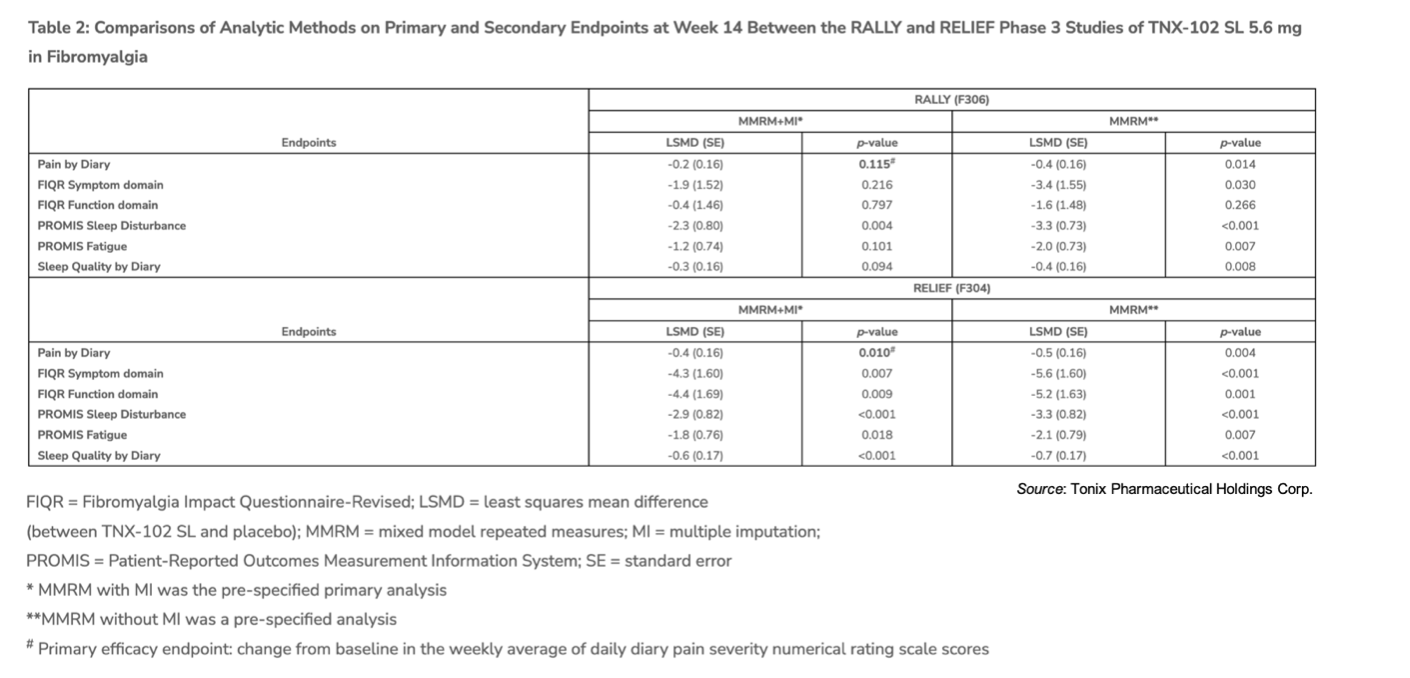
To account for missing data (i.e., when a patient discontinues the data that would have been collected for them is considered ‘missing’), the statistical analysis is performed using a method called ‘multiple imputation’ (MI), which ultimately results in a negative impact from the missing data. For example, the following table shows the results when performing the statistical analysis with and without MI. Without MI, the results of the RELIEF and the RALLY trials appear consistent.
What could account for the dramatic increase in discontinuations in the RALLY study? While there are many potential reasons, one notable difference between the RALLY and RELIEF studies is that the RALLY study was taking place during the height of the COVID-19 pandemic when vaccines were being administered across the country. This may have contributed to a more challenging environment for participants in the trial.
Regardless, the company will now turn its attention to the Phase 3 RESILIENT study now that the COVID-19 pandemic is entering the endemic phase, which will hopefully lead to adverse event-related discontinuations returning to levels seen in the RELIEF and posttraumatic stress disorder (PTSD) studies. We anticipate the RESILIENT trial initiating in the first half of 2022.
Multiple Clinical Trials to Initiate Over the Next Year
Tonix has built a diverse pipeline that includes development candidates for COVID, biodefense, immunology, and multiple central nervous system (CNS) diseases. The company is expected to initiate a clinical trial for TNX-1300 in the near term, with multiple other trials initiating for other development candidates over the next year:
• TNX-1300: The company will be initiating a Phase 2 clinical trial of TNX-1300 for the treatment of cocaine overdose in the first half of 2022. TNX-1300 is a recombinant enzyme derived from the CocE gene of a Rhodococcus species that utilizes cocaine as a sole source of carbon and nitrogen (Bresler et al., 2000). Results from a previous Phase 2 clinical trial showed that the recombinant CocE enzyme (then called RBP-8000, now TNX-1300) rapidly degraded plasma cocaine levels in volunteer cocaine users and was safe and well tolerated.
• TNX-102 SL: Phase 3 RESILIENT trial in FM: Initiation – 1H22
• TNX-102 SL: Phase 2 trial in PTSD (in Kenya): Initiation – 1H22
• TNX-102 SL: Phase 2 trial in Long COVID: Initiation – 1H22
• TNX-1900: Phase 2 trial for chronic migraine: Initiation – 2H22
• TNX-601 CR: Phase 2 trial in Major Depressive Disorder: Initiation – 1Q23
• TNX-2100: First-in-human trial: Topline Data – 1H23
Financial Update
On March 14, 2022, Tonix announced financial results for the fourth quarter and full year 2021. As expected, the company did not report any revenues for the fourth quarter or full year 2021. Net loss for the fourth quarter of 2021 was $29.6 million, or $0.07 per share, compared to a net loss of $17.0 million, or $0.10 per share, for the fourth quarter of 2020. The weighted average common shares outstanding for the fourth quarter of 2021 were approximately 451.2 million compared to approximately 163.9 million for the fourth quarter of 2020.
R&D expenses for the fourth quarter of 2021 were $22.3 million, compared to $12.1 million for the fourth quarter of 2020. The increase was primarily due to increased clinical expenses, manufacturing expenses, non-clinical expenses, and employee-related expenses. G&A expenses for the fourth quarter of 2021 were $7.3 million, compared to $4.9 million for the fourth quarter of 2020. The increase was primarily due to an increase in employee-related expenses
For 2021, net loss available to stockholders was $92.3 million, or $0.26 per share, compared to a net loss of $52.2 million, or $0.55 per share, for 2020. The weighted average common shares outstanding for full year 2021 was approximately 360.2 million, compared to approximately 94.6 million for full year 2020. R&D expenses in 2021 were $68.8 million, compared to $36.2 million for 2020. The increase was primarily due to increased non-clinical expenses, manufacturing expenses, employee-related expenses, and regulatory/legal expenses. G&A expenses for 2021 were $23.5 million, compared to $14.4 million for 2020. The increase is primarily due to increased employee-related expenses, legal fees, investor relations expenses, and financial reporting expenses.
As of December 31, 2021, Tonix had approximately $178.7 million in cash and cash equivalents. Subsequent to the end of the year, the company sold 15.6 million shares of common stock in at-the-market (ATM) sales under a sales agreement with A.G.P. for net proceeds of approximately $4.3 million. In addition, the company sold 22.0 million shares under the purchase agreement with Lincoln Park for net proceeds of approximately $4.5 million. We estimate the company currently has sufficient capital to fund operations through the end of 2022. As of March 14, 2022, Tonix had approximately 533.9 million shares outstanding.
Conclusion
While it’s unfortunate that the RALLY study did not meet the primary endpoint, we are glad to see Tonix moving ahead with the RESILIENT trial, as just one more successful Phase 3 trial is necessary before an NDA can be filed for TNX-102 SL in fibromyalgia. The company’s move into monoclonal antibody development with TNX-1500 will be interesting to watch, as those assets typically draw partnering interests earlier than other types of compounds. Tonix recently raised $8.8 million from stock sales, and after accounting for the increased share count our valuation has decreased to $1.50.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.