By David Bautz, PhD
NASDAQ:MDNA
READ THE FULL MDNA RESEARCH REPORT
Business Update
Update on ABILITY Trial Expected in Calendar 3Q23
Medicenna Therapeutics Corp. (NASDAQ:MDNA) is currently conducting the Phase 1/2 ABILITY Study (A Beta-only IL-2 ImmunoTherapY Study) of MDNA11 in patients with advanced solid tumors (NCT05086692). In the ongoing dose escalation portion of the study, MDNA11 is administered intravenously once every two weeks to patients with advanced solid tumors. The first two cohorts evaluated MDNA11 at doses ≤ 10 µg/kg. The third cohort was administered a dose of 30 µg/kg. Patients in the fourth and fifth cohorts received two 30 µg/kg “priming” doses of MDNA11 before stepping up to receive fixed doses of 60 and 90 µg/kg, respectively. Cohort six will receive a target dose of 120 µg/kg following three priming doses of 30, 60, and 90 µg/kg. This is summarized in the following figure.
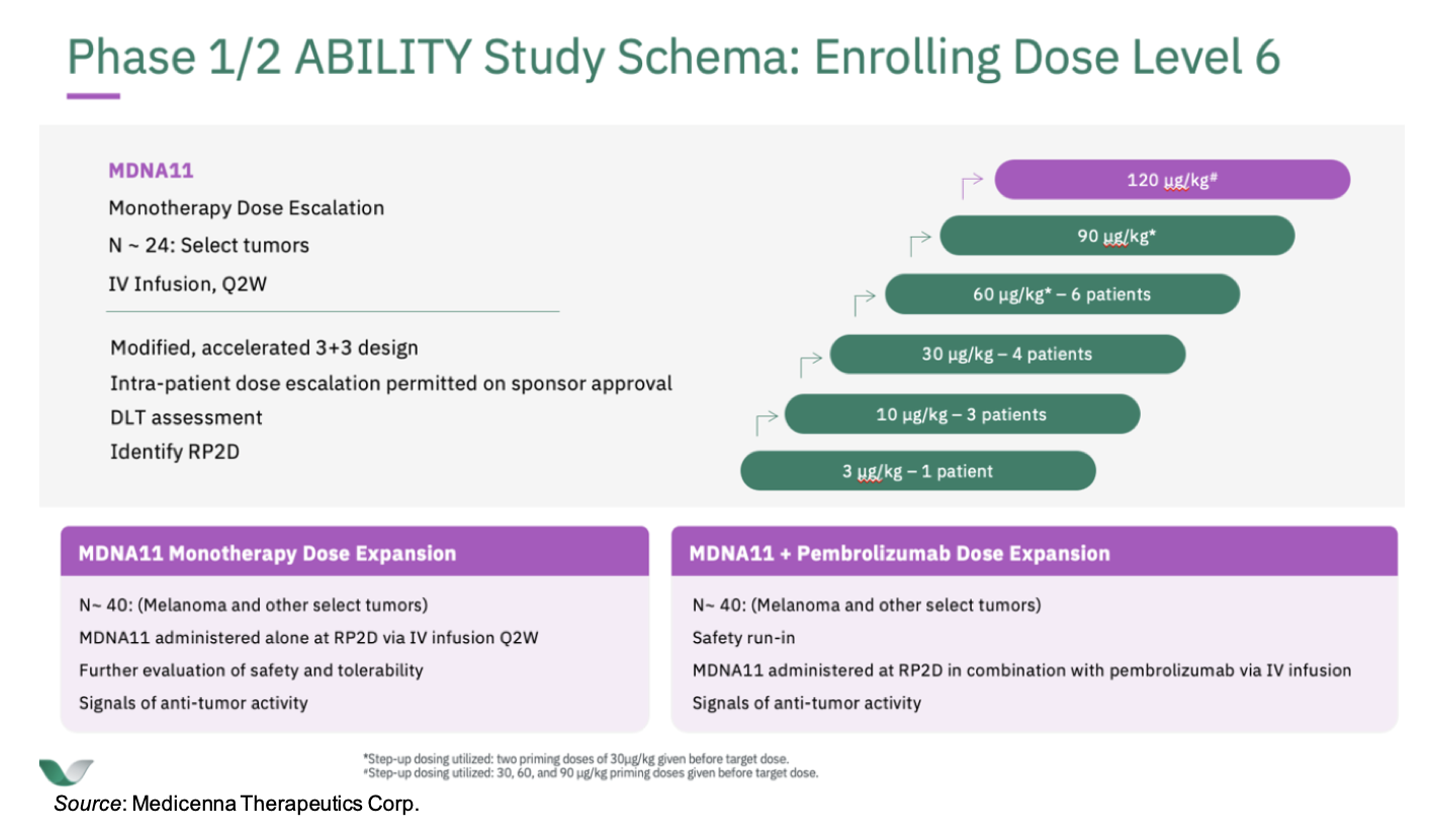
The company is currently enrolling patients in the sixth and final dose escalation cohort, which we expect will complete in mid-calendar year 2023. Following that, we anticipate a comprehensive update on PK, PD, safety, and efficacy from all six cohorts, which will include initial anti-tumor activity data from the fifth and sixth dose escalation cohorts in calendar 3Q23. We expect the Phase 2 monotherapy dose expansion portion of the trial to commence in calendar 3Q23 and the combination therapy (MDNA + Keytruda) to initiate in calendar 4Q23.
Key Data from the first four dosing cohorts includes:
Safety:
• There were no dose-limiting toxicities in Cohort 4
• There have been no significant increases in eosinophil count from baseline associated with MDNA11 treatment. This is important as high eosinophil count is associated with vascular leak syndrome, a serious side effect that is known to occur with high-dose recombinant human IL-2 (Proleukin®)
PK/PD:
• Dose dependent increases in Cmax and Area Under the Curve were observed
• No sign of immunogenicity as exhibited by a lack of anti-drug antibodies
• MDNA11 preferentially expanded anti-cancer NK and CD8+ T cells without stimulating proliferation of pro-tumor Treg cells
Anti-Tumor Activity:
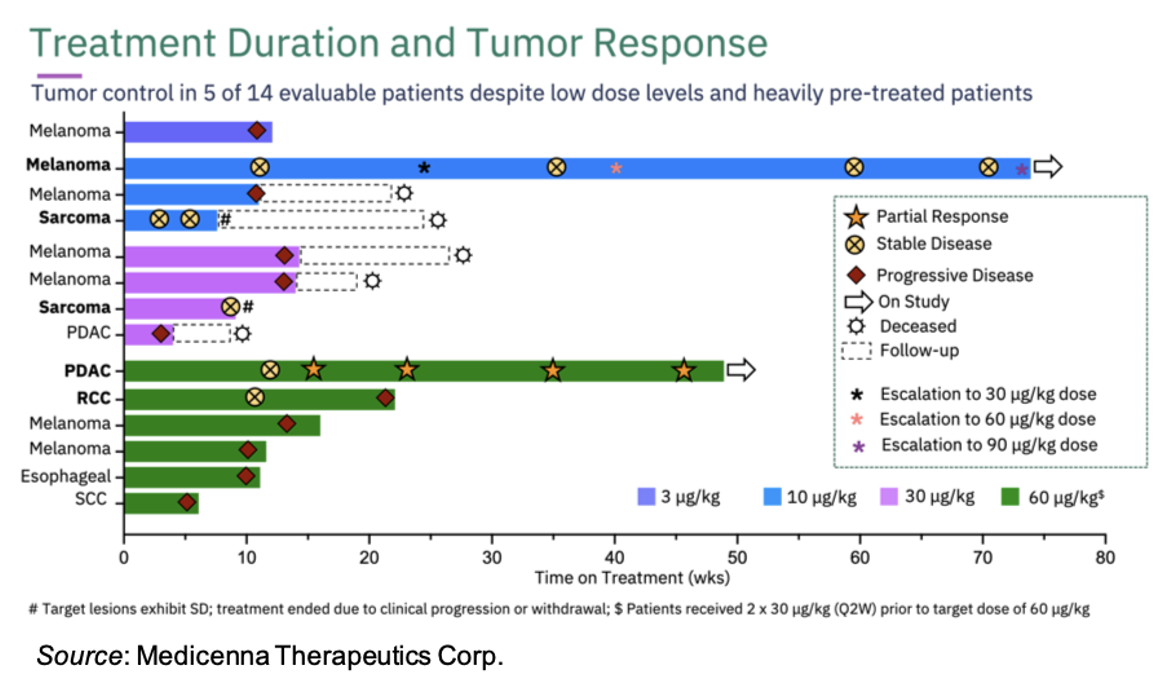
Of the 14 evaluable patients, five achieved tumor control (stable disease, partial response, or complete response as per RECIST 1.1):
1. Metastatic Leiomyosarcoma Stage IV (Dose Level 2 @ 10 µg/kg); stable disease.
2. Metastatic Melanoma Grade 4C (initially enrolled at Dose Level 2 @ 10 µg/kg Q2W with subsequent intra-patient dose escalations to Dose Level 3 @30 µg/kg and Dose Level 4 @ 60
μg/kg), stable disease.
3. Metastatic Sarcoma Stage IV (Dose Level 3 @ 30 µg/kg), stable disease
4. Pancreatic Ductal Adenocarcinoma (PDAC) Stage IV (Dose Level 4 @ 60 µg/kg following 2 priming
doses of 30 µg/kg), confirmed partial response.
5. Non-clear cell 3L renal cell carcinoma patient (Dose Level 4 @ 60 µg/kg following 2 priming doses of 30 µg/kg), stable disease.
A summary of the treatment duration and responses for each of the 14 evaluable patients is shown below:
New Preclinical Data on IL-13 Superkines Presented at AACR
In April 2023, Medicenna announced that new preclinical data characterizing the Interleukin-13 (IL-13) Superkines MDNA132 and MDNA213, along with a series of next-generation IL-13 Superkines, was presented at the 2023 Annual Meeting of the American Association for Cancer Research (AACR) (Sharma et al., 2023). IL-13 signals through two different receptors; The Type II receptor, which is composed of IL-4Rα/IL-13Rα1, and the IL-13Rα2 decoy receptor, whose exact function is unclear. IL-13 binding to the Type II receptor activates the Stat6 signaling pathway that ultimately promotes M2 tumor associated macrophages (TAMs) and myeloid derived suppressor cells (MDSCs). Both of these cell types limit immune effector cells and promote an ‘immunologically cold’ tumor microenvironment (Bhattacharjee et al., 2013).
MDNA132 and MDNA213 target the IL-13Rα2 decoy receptor, which is overexpressed in a number of different cancer types (pancreatic, prostate, colorectal, etc.) but has little to no expression in normal tissues. In addition, increased expression of IL-13Rα2 correlates with cancer invasion, metastasis, and poor survival. Thus, IL-13Rα2 has become an attractive cancer target given its tumor specificity and high expression in immune suppressed “cold tumors”.
Both MDNA132 and MDNA213 were designed to have increased affinity to IL-13Rα2 and no binding to IL-13Rα1. Directed evolution of IL-13 resulted in the selection of MDNA132, which exhibits no binding to IL-13Rα1 but also slightly decreased binding to IL-13Rα2. Additional mutations designed through in-silico modeling resulted in MDNA213, which also exhibits no binding to IL-13Rα1 but has increased affinity to IL-13Rα2 even when compared to native IL-13.
Medicenna is designing a family of next-generation IL-13 Superkines that combine the IL-13 binders with other molecules for enhanced anti-tumor activity or targeted delivery of potent toxins or immune modulators. The different IL-13 Superkines the company is developing is shown in the following figure.
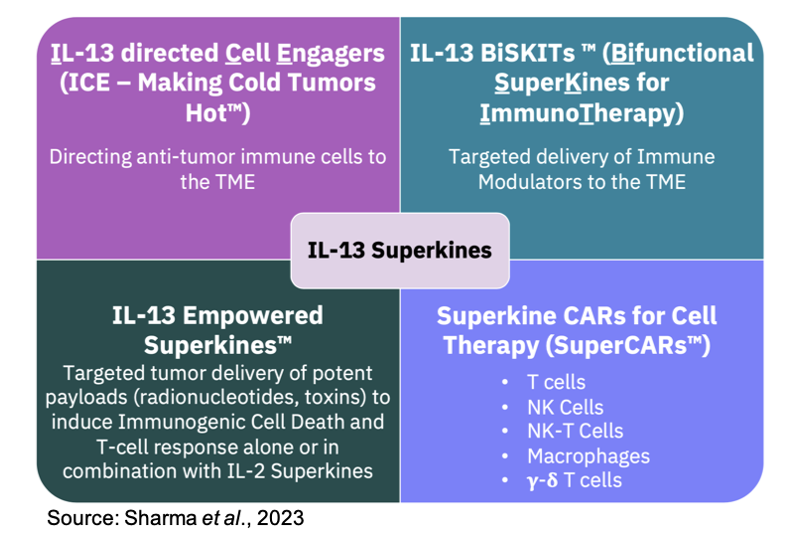
The presentation at AACR included data for a series of next-generation IL-13 Superkines:
Anti-mCD3-MDNA132: This is a first-in-class IL-13 directed Cell Engager (ICE-Making Cold Tumors Hot™) that is comprised of MDNA132 fused to an anti-CD3 antibody. The in vitro data showed that the molecule retains high affinity to IL-13Rα2, which is expressed on tumor cells, and CD3, which is expressed on immune cells. The theory behind this molecule is that it will recruit the patient’s own immune cells to the tumor microenvironment to directly target immunologically “cold” tumors.
MDNA19-MDNA213: This is a first-in-class BiSKIT™ (Bifunctional SuperKines for ImmunoTherapy) that is comprised of MDNA213 fused to MDNA19, the Fc version of MDNA11. The in vitro data show that this BiSKIT retains strong binding to both IL-13Rα2 and CD122 (IL-2Rß) while stimulating effector T cells and NK cells without stimulating Tregs.
Anti-mPD1-MDNA213: This is a first-in-class BiSKIT that is comprised of MDNA213 fused to an anti-PD-1 antibody. The in vitro data show that the molecule retains strong binding to both IL-13Ra2 and PD1 while effectively blocking the PD1/PD-L1 interaction with a similar potency as the native anti-PD1 antibody.
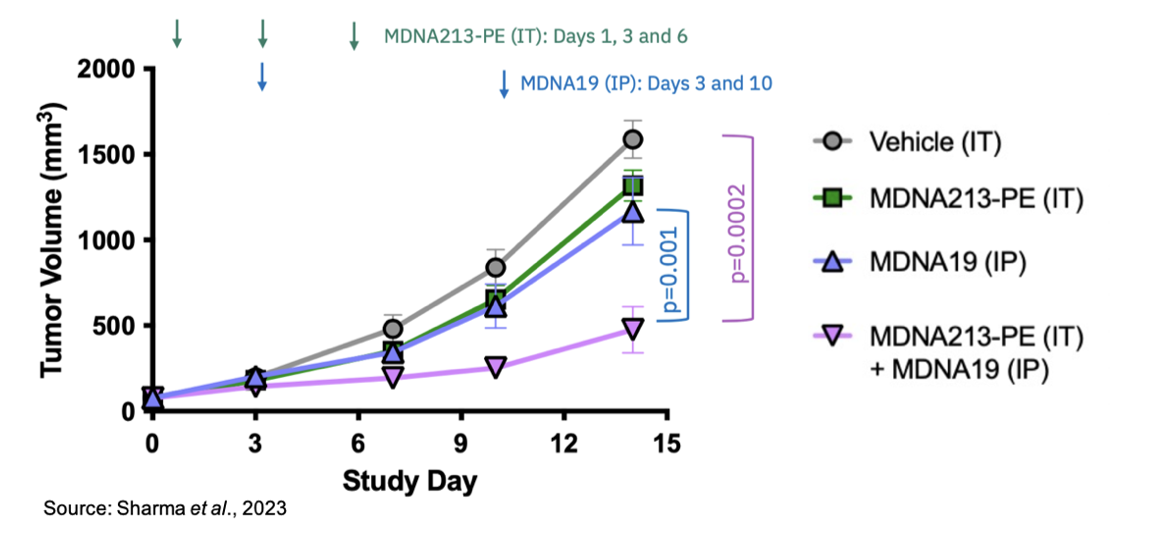
MDNA213-PE: This is an Empowered Superkine that is comprised of MDNA213 fused to the PE cytotoxin (same cytotoxin utilized in bizaxofusp, formerly MDNA55). The in vitro data show that the molecule exhibits potent cytotoxicity against human and murine cancer cells that express IL-13Rα2. In addition, as the following image shows, in a murine triple-negative breast cancer model, MDNA213-PE inhibited tumor growth as both a single agent and in combination with MDNA19.
Financial Update
On June 27, 2023, Medicenna announced financial results for fiscal year 2023, which ended March 31, 2023. As expected, the company did not report any revenues for fiscal year 2023. Net loss was CAD$10.0 million, or $0.16 per share, compared to a net loss of CAD$22.6 million, or $0.42 per share, for the fiscal year ending March 31, 2022. R&D expenses for fiscal year 2023 were approximately CAD$9.3 million, compared to approximately CAD$14.7 million for fiscal year 2022. The decrease was primarily due to costs associated with the development of MDNA11 incurred in fiscal year 2022, which included GMP manufacturing and IND enabling studies. G&A expenses in fiscal year 2023 were CAD$7.0 million, compared to CAD$7.8 million for fiscal year 2022. The decrease was primarily due to reduction in directors and officers liability insurance premiums.
As of March 31, 2023, Medicenna had approximately CAD$33.6 million in cash and cash equivalents. We estimate that the company is funded through key milestones in the ABILITY trial and through calendar 3Q24. As of June 27, 2023, Medicenna had approximately 69.6 million shares of common stock outstanding and, when factoring in warrants and stock options, a fully diluted share count of approximately 91.4 million.
Conclusion
The data reported thus far for the ABILITY trial has been very encouraging and we are looking forward to the comprehensive update in the third calendar quarter of 2023, which we expect will include preliminary anti-tumor activity for Cohorts five and six. While preliminary, the data presented at AACR shows the potential for the IL-13 Superkine platform and we look forward to additional updates on this and other preclinical programs the company has ongoing. With no changes to our model our valuation remains at $7.00 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.