By David Bautz, PhD
OTC:MSCLF
READ THE FULL MSCLF RESEARCH REPORT
Business Update
SAT-3153 Selected as Lead Development Candidate
On January 3, 2023, Satellos Bioscience Inc. (OTC:MSCLF) announced the designation of SAT-3153 as its lead development candidate. SAT-3153 is a protein kinase inhibitor that targets a particular protein in the Notch signaling pathway (codenamed “K9”). Following results obtained through genetic ablation of K9, Satellos hypothesized that targeting K9 could modulate asymmetric muscle stem cell division. Since K9 is already the target of other pharmaceutical interventions in a different disease setting, Satellos was able to synthesize existing K9 inhibitors to test the hypothesis. These studies showed that inhibiting K9 resulted in modulation of muscle stem cell division, enhanced muscle regeneration, and increased muscle mass/function.
We anticipate IND-enabling studies for SAT-3153 commencing soon and we look forward to updates from the company this year.
Preclinical Studies Support Targeting K9
Satellos recently provided additional preclinical data supporting the targeting of K9, which can be found on its updated investor presentation here. Using a compound that is already under clinical investigation that targets K9, Satellos performed a number of preclinical proof-of-concept experiments to determine the effect of inhibiting K9 on muscle stem cell polarity and division. It is important to remember that the following results were not performed with SAT-3153, but with an entirely separate compound that also targets K9.
The following image shows the result of treating Mdx mice, which are used as a model for studying Duchenne muscular dystrophy (DMD), with the K9 inhibitor. The graph on the left shows that following treatment of the mice there is no change in the number of muscle stem cells between mice treated with drug and those treated with placebo. The graph on the right shows that mice treated with drug had a significantly higher percentage of progenitor cells than mice treated with placebo, suggesting that the drug is pushing the muscle stem cell population toward asymmetric division. As a reminder, in patients with DMD it is the lack of asymmetric division in muscle stem cells that ultimately leads to muscle degeneration, thus any treatment that can increase the number of asymmetric divisions could lead to a restoration of muscle tissue and function.

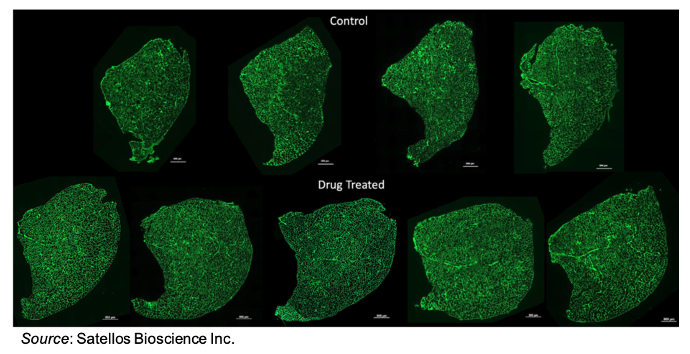
The following images show a cross section of the same muscle from multiple mice. The mice treated with drug show a much larger cross-sectional area than the mice treated with placebo. This is indicative of the K9 inhibitor leading to an increase in muscle mass. This could represent an exciting breakthrough in DMD research, as new muscle growth would be essential to not just stopping progression of the disease but also being able to potentially reverse its effects.
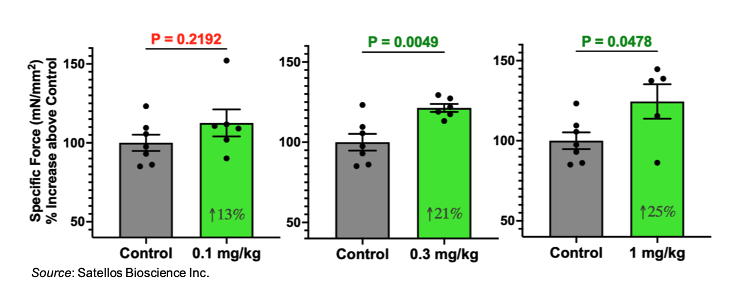
Lastly, the increase in muscle size shown above is also accompanied by a dose-dependent increase in muscle force. The following graphs show the specific muscle force for drug-treated and placebo-treated mice. As the dose of drug increases, those mice experience a proportionate increase in their muscle force. These results indicate that not only are the mice generating muscle tissue, but that this muscle tissue is functional and leads to an increase in muscle strength.
We believe the aforementioned results represent just a small portion of the data Satellos has obtained showing that inhibition of K9 results in positive effects on muscle stem cell division, muscle growth, and muscle strength. We anticipate similar studies being performed with SAT-3153 and we look forward to the results of those studies later in 2023.
Financial Update
In November 2022, Satellos announce financial results for the third quarter of 2022. The company did not have any revenues in the third quarter of 2022. Expenses for the third quarter of 2022 consisted of:
• R&D expenses net of refundable tax credits was approximately CAD$0.6 million in the third quarter of 2022 compared to approximately CAD$0.5 million for the third quarter of 2021. The increase was due to the company continuing to increase R&D contractor spending.
• Management fees and salaries was approximately CAD$0.5 million for the third quarter of 2022 compared to approximately CAD$0.3 million for the third quarter of 2021. The increase was due to increased salaries and consulting fees.
• Professional fees were approximately CAD$0.3 million for the third quarter of 2022 compared to approximately CAD$0.6 million for the third quarter of 2021. The decrease was due to lower legal fees.
• G&A expenses were approximately $0.3 million for the third quarter of 2022 compared to CAD$0.1 million for the third quarter of 2021. The increase was due to increased depreciation and amortization due to amortization of the technology acquired from iCo therapeutics following the reverse merger along with higher insurance costs and travel expenses.
• Stock-based compensation was approximately CAD$0.3 million for the third quarter of 2022 compared to CAD$15,447 for the third quarter of 2021. The increase was due to a larger number of options granted during the current period.
As of September 30, 2022, Satellos had approximately CAD$3.1 million in cash and cash equivalents. We estimate that the company has sufficient capital to fund operations into the second quarter of 2023. As of September 30, 2022, Satellos had approximately 41.8 million shares outstanding and, when factoring in stock options and warrants, a fully diluted share count of approximately 51.0 million.
Conclusion
Having now identified SAT-3153, we believe Satellos will attempt to recapitulate the above results with that compound as part of its IND-enabling work. We anticipate an IND filing with the FDA by the end of 2023 or in early 2024 such that clinical trials can commence. The results obtained with inhibition of K9 could represent a true turning point in DMD research, as the ability to stimulate muscle growth may result in a halt to disease progression or even reversal of damage in DMD patients. We look forward to additional updates as the company moves closer to initiating its first-in-human studies in 2024. Our valuation remains at $0.70.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.