By John Vandermosten, CFA
NASDAQ:ACHV
READ THE FULL ACHV RESEARCH REPORT
2Q:24 Operational and Financial Results
Achieve Life Sciences, Inc. (NASDAQ:ACHV) reported second quarter 2024 results in an August 13th press release distributed after the market’s close. The company subsequently held a conference call discussing progress with the ORCA OL trial and in the vaping indication. Form 10-Q was filed with the SEC providing additional disclosures. Highlights during the second quarter and to date include presentation of vaping trial results in multiple scientific venues, agreement with the FDA on the structure of a study to evaluate long term exposure to cytisinicline, rapid and successful initial enrollment in the ORCA OL trial, loan refinancing with Silicon Valley Bank and a Breakthrough Therapy Designation for the vaping indication.
Milestones:
➢ Presentation at Life Science Innovation Northwest 2024 – April 2024
➢ Launch of ORCA-OL trial – May 2024
➢ Addition to the Russell 3000 and Microcap Indices – July 2024
➢ Refinancing of SVB load – July 2024
➢ Grant of Breakthrough Therapy Designation for vaping indication – July 2024
➢ Over 650 enrolled in the ORCA OL trial – August 2024
➢ End of Phase II meeting with FDA to determine Phase III vaping cessation trial design – 2H:24
➢ Data from 300 patients with six months exposure to cytisinicline – year-end 2024
➢ Submission of new drug application (NDA) – 1H:25
➢ Data from 100 patients with twelve months exposure to cytisinicline – mid-year 2025
➢ Launch of Phase III vaping trial – Mid-2025
➢ FDA target action date for cytisinicline NDA – 1H:26
Financial Results
No revenues were reported for 2Q:24. Operating expense was $8.4 million producing a net loss of ($8.5) million or ($0.25) per share. For the quarter ending June 30, 2024 and versus the same comparable period in the prior year:
➢ Research & development expense totaled $5.1 million, up 12% from $4.6 million, due to the initiation and enrollment of the ORCA-OL open label safety trial in May 2024;
➢ General & administrative expense was $3.3 million, up 6% from $3.1 million on stock-based compensation and consulting costs. The increased expenses were partially offset by a decrease in legal costs associated with general corporate activities;
➢ Net other expense was ($30,000) vs. ($525,000) due to greater interest income on higher cash balances;
➢ Net loss was ($8.5) million vs. ($8.2) million or ($0.25) and ($0.43) per share, respectively.
As of June 30th, 2024, cash and equivalents totaled $61.3 million. This amount compares to a $15.5 million balance in cash and equivalents held at the end of 2023 with the increase due to the February 2024 equity raise. Achieve carries convertible debt of $17.6 million on the balance sheet which includes accrued interest. The company refinanced its debt agreement with Silicon Valley Bank (SVB), extending maturity until the end of 2027. Achieve is eligible to draw an additional $5 million from the facility following FDA acceptance of its new drug application (NDA). Cash used in operations during the first half was ($10.2) million versus ($15.0) million in the same prior year period.
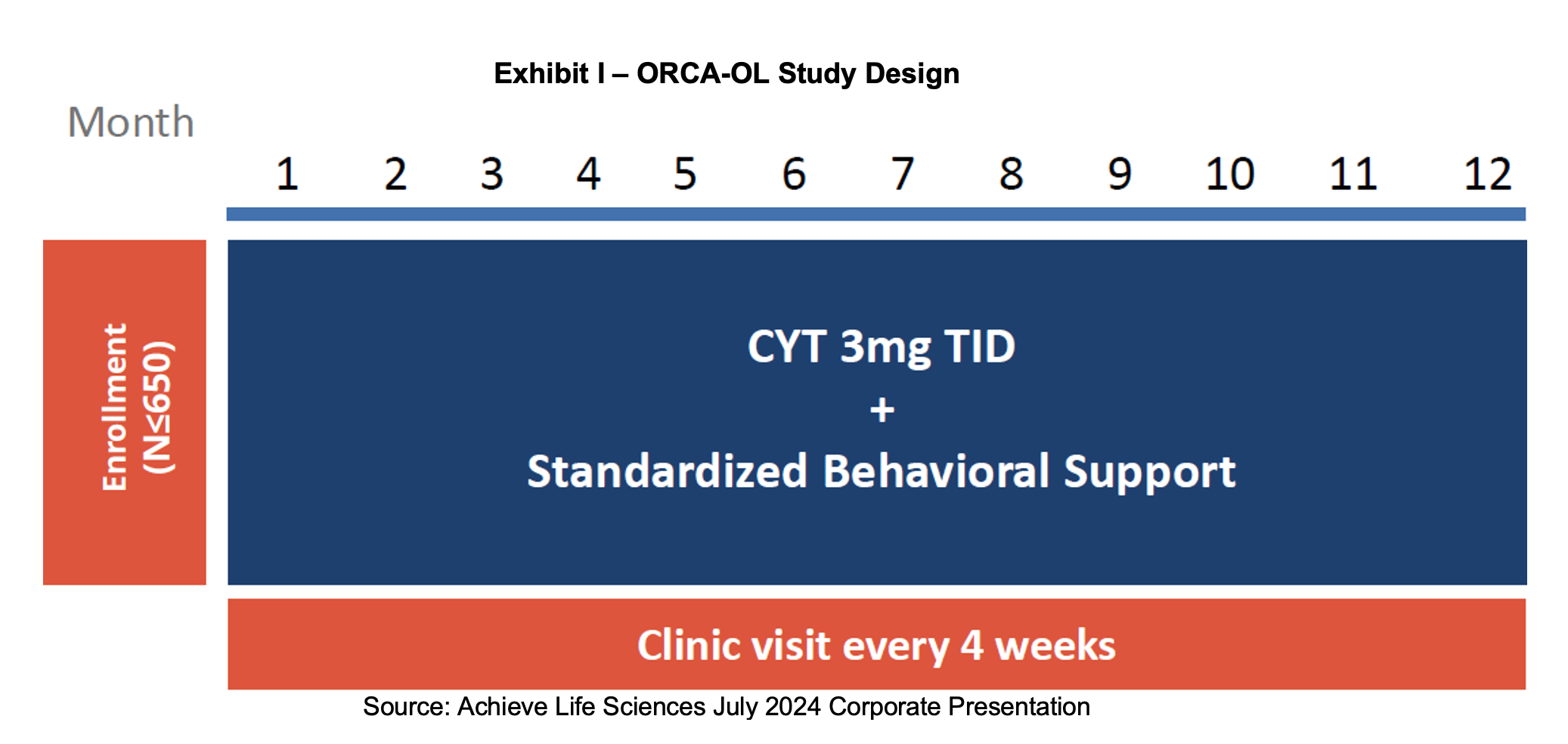
ORCA-OL Trial
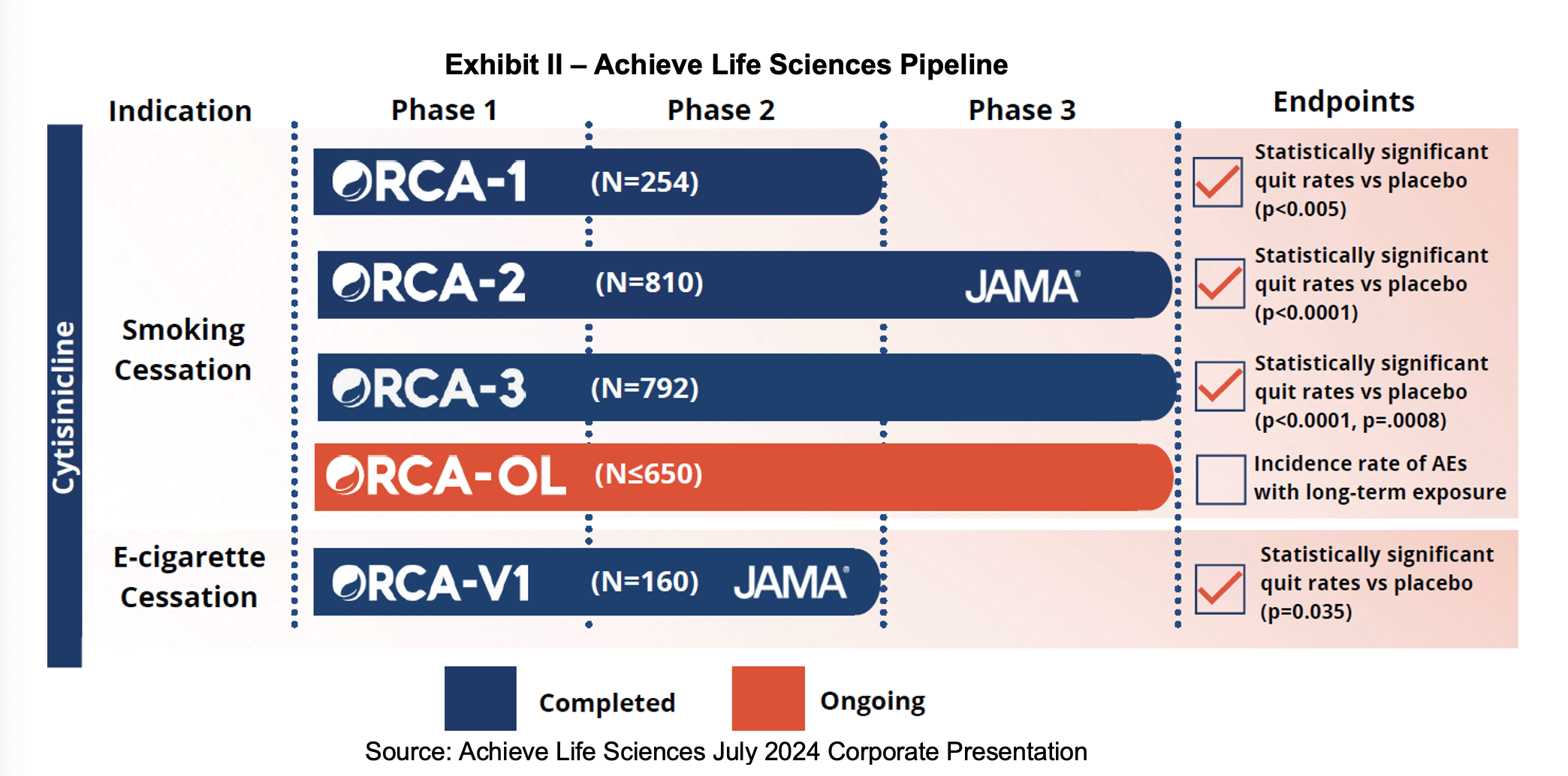
During the 2Q:24 call, Achieve provided an update on enrollment in the Ongoing Research of Cytisinicline for Addiction Program, Open Label (ORCA-OL) trial. After a launch in May, the trial has started off with rapid enrollment, high screening success rates and a single digit dropout rate. More than 650 subjects have been enrolled with about two thirds already having six or twelve weeks of treatment and one third from the placebo group in a prior ORCA trial. 29 sites are actively enrolling with the objective of obtaining six months of safety data from 300 of these individuals in support of the anticipated filing of the NDA in 1H:25. If retention rates remain high, Achieve may close out the trial early in order to conserve capital. While the FDA will not consider any efficacy data, Achieve will measure subsequent treatment success which may inform providers if cytisinicline is ultimately approved.
The ORCA OL trial arose from the FDA’s desire for additional long-term cytisinicline exposure data to adequately assess safety risk. Despite an initial anticipated treatment duration of six to twelve weeks, cytisinicline could be used for chronic, repeat or intermittent use if a patient relapses. With this possibility guiding its interactions with drug sponsors, the FDA and Achieve reached an agreement that a single, open-label study evaluating long-term safety exposure of cytisinicline will meet the safety requirement. Details of the arrangement were provided in a February 29th press release and are described further below.
The completed study will include safety data on at least 300 subjects that have received cumulative cytisinicline treatment for six months. This data will be included with the new drug application (NDA), which we expect in the first half of 2025. Additionally, one year of exposure safety data from at least 100 subjects treated with cytisinicline are required to be submitted prior to approval. Subjects for the trial will be drawn from the pool of individuals that participated in the ORCA-1, ORCA-2 and ORCA-V1 trials with a preference for subjects who received 12 weeks of treatment. The desired exposure data is cumulative rather than continuous, giving credit to the duration of therapy already received.
The ORCA-OL trial will recruit from the more than 1,700 subjects who have already participated in Achieve’s previous studies. 1,100 of these subjects have been treated with six or twelve weeks of cytisinicline. Exit data from the ORCA studies have shown that patients are largely satisfied with cytisinicline and the drug is well tolerated. One notable takeaway from interactions the investigators have had with former trial participants is that 25% of them are not eligible for the trial as they are no longer using nicotine products.
Vaping Indication
Breakthrough Therapy Status
At the end of July, Achieve announced that the FDA had granted Breakthrough Therapy status for cytisinicline for the treatment of e-cigarette or vaping nicotine dependence. The designation is designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint. The status provides for expedited development where Achieve can receive intensive guidance on drug development, have close contact with the senior managers and review staff at the agency, participate in rolling review and potentially be eligible for priority review, which can shave some time off the approval process.
Management would like to leverage this expedited status to obtain a label that allows for adolescent use as this demographic uses vaping products at a much higher rate than older individuals. Since the announcement of the status grant, Achieve has requested a Type B end of Phase II meeting where the groups will begin discussions for designing a pivotal trial. The team anticipates starting the single required Phase III vaping trial in the middle of 2025.
Summary
Achieve has shown impressive safety and efficacy in its Phase III trials and is in the last stages of preparing for submission of an NDA. The FDA’s request for a long-term safety study has materialized into ORCA-OL which has successfully launched and enrolled at a better-than-expected pace. Now that the company has everything in place to generate and prepare the necessary data for a successful NDA submission, we will watch closely to see if some of the timelines can be narrowed from the 1H:25 submission target. Efforts to move forward the pivotal vaping trial are ongoing. The FDA granted Breakthrough Therapy status in July which can help accelerate the pace, reduce the cost and increase the probability of success of obtaining a vaping indication.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.