By John Vandermosten, CFA
NASDAQ:ACHV
READ THE FULL ACHV RESEARCH REPORT
Achieve Life Sciences, Inc. (NASDAQ:ACHV) announced that its new drug application (NDA) for cytisinicline has been accepted by the FDA in a September 3rd press release. Other important developments include the appointment of two internal employees to interim Chief Medical Officer (CMO) and Chief Operations Officer (COO). The promotions reflect a broader change to the executive ranks as the company prepares to transition from a development company to a commercial organization. Achieve has also participated in two September conferences in New York, providing the opportunity for investors to receive an update on the company’s progress and conduct 1:1 meetings.
NDA Submission and Acceptance
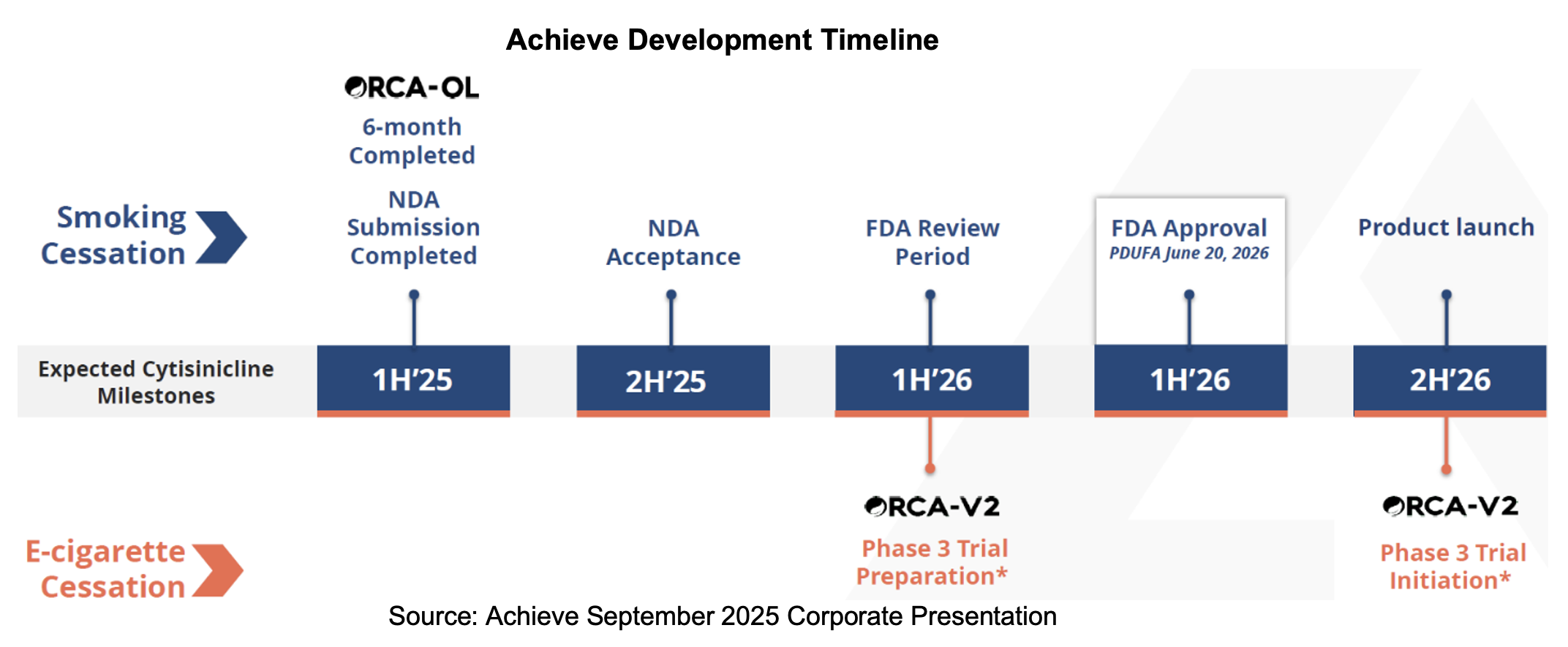
Achieve announced its NDA submission of cytisinicline for smoking cessation in a June 26th press release. On September 3rd, the company reported FDA acceptance of its NDA and the assignment of a June 20th, 2025, Prescription Drug User Fee Act (PDUFA) date. Cytisinicline completed two Phase III studies, an open label safety study, and other studies that evaluated over 2,000 participants, with the results demonstrating its safety, efficacy, and tolerability. The other outstanding regulatory milestone is the submission of the one-year ORCA-OL safety data, which is expected to be shared with the agency in October.
Appointments and Promotions
Achieve announced the appointment of Dr. Mark Rubinstein as interim Chief Medical Officer (CMO) and the promotion of Craig Donnelly to Chief Operations Officer (COO). Dr. Rubinstein, M.D. succeeds Cindy Jacobs, Ph.D., M.D. in the role. Dr. Rubinstein was hired as Head of Medical Affairs at Achieve in October 2024, having previously served as Head of Medical Affairs at Blip, a smoking cessation company. Dr. Jacobs is expected to provide advisory services as the new CMO ramps up in the role. Craig Donnelly joined Achieve in 2022 as Executive Vice President of Chemistry, Manufacturing, and Controls (CMC). As COO, Craig will lead the integration of Achieve’s supply chain and manufacturing activities with its commercial strategy.
Milestones
- Development of cytisinicline product label for smoking cessation – 1H:25
- Completion of six months of ORCA-OL safety data for 300 subjects – January 2025
- Selection of 3rd party logistics partner – 2Q:25
- NDA Submission – 2Q:25
- BTIG Virtual Biotechnology Conference attendance – July 2025
- Anticipated acceptance of NDA by FDA – September 2025
- Attendance at HC Wainwright conference – September 2025
- Attendance at Lake Street Conference – September 2025
- FDA data submission from patients with twelve months of exposure to cytisinicline – 4Q:25
- Launch of Phase III vaping trial – 1H:26
- FDA target action date for cytisinicline NDA – June 20th, 2026
- Launch of cytisinicline – Late 2026
Summary
Achieve surpassed an important hurdle as the FDA accepts cytisinicline’s NDA and assigns a PDUFA date of June 20th, 2026. The company also raises two internal employees to CMO and COO, strengthening the team as it advances towards commercialization. The Achieve team is on the road, sharing the investment thesis with stakeholders, specifically with investors at the Lake Street and HC Wainwright conferences in New York. We expect to hear updates on progress and details on the submission of one-year safety data during the third quarter conference call expected in mid-November.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.