By John Vandermosten, CFA
NASDAQ:BLRX
READ THE FULL BLRX RESEARCH REPORT
BioLineRx Ltd. (NASDAQ:BLRX) reported third quarter 2025 results, producing license revenues of $427,000. Following the September announcement of the joint venture (JV) with Hemispherian, the JV has been preparing for its Phase I/IIa study for GLIX1 in GBM in 1Q:26. GLIX1 received a Notice of Allowance from the USPTO for a patent which we expect will soon be granted. BioLineRx provided select data from its study with Washington University that evaluated motixafortide in stem cell mobilization for sickle cell disease (SCD) patients. The company’s partnership with Columbia University continues with an interim readout expected for the pancreatic ductal adenocarcinoma (PDAC) study in 2026 and full enrollment by 2027.

BioLineRx reported 3Q:25 sales of $427,000 producing a net loss from operations of $2.2 million or $0.00 per share. Non-operating income offset $1.2 million of the operating loss producing a net loss of $1.0 million or $0.00 per share. The results were announced in a press release on November 24th, 2025, followed by a conference call with management and the filing of Form 6-K providing additional information.
Below we summarize financial results for the three-month period ended September 30th, 2025, compared to the same prior year period:
- Total and license revenues were $427,000 from the sale of Aphexda compared to $4.9 million related to the out-licensing transaction with Gloria and direct commercial sales. Ayrmid’s Aphexda product sales were $2.4 million;
- Cost of revenues was $84,000 which largely represents a pass-through to license-holder Biokine as a royalty on motixafortide revenues vs. $822,000;
- Research and development expenses totaled $1.7 million, down 33% from $2.6 million, with the decline attributable to lower expenses related to motixafortide due to the out-licensing of rights to Ayrmid and a reduction in compensation arising from lower headcount;
- Sales and marketing expenses were $0.0 vs. $5.6 million due to the shutdown of U.S. commercial operations in the fourth quarter of 2024 following the Ayrmid out-licensing transaction;
- General and administrative (G&A) expenses were $831,000, down 40% from $1.4 million as a result of lower headcount and a decrease in a variety of other miscellaneous expenses;
- Non-operating income was $1.2 million vs. $0.8 million reflecting changes in fair-value adjustments of warrant liabilities on the balance sheet;
- Net financial income amounted to $73,000 reflecting interest income exceeding interest expense;
- Net loss was $1.0 million compared to $5.8 million, or $0.00 per share in each period.
Cash, equivalents and short-term bank deposits as of September 30th, 2025 totaled $25.2 million, up from the year end 2024 balance of $19.6 million. Cash burn for the first nine months of 2025 was ($4.9) million and net cash from financing was $10.1 million. $2.4 million in cash from the Gloria milestone was received in June. Financing cash contributions came from issuance of share capital and warrants as well as net proceeds from the ATM agreement with H.C. Wainwright. The ATM raised $5.0 million year to date as of the third quarter end. As of September 30th, debt was carried at $10.1 million on the balance sheet. This was partially offset by repayment of debt. The term loan is expected to be fully repaid by the end of 2027.
Poster Presentation at American Society of Hematology (ASH) Annual Meeting
BioLineRx entered into a clinical collaboration with the Washington University School of Medicine in St. Louis to conduct a Phase I study in March of 2023. The trial evaluated motixafortide as a monotherapy and as a combination therapy with natalizumab to mobilize CD34+ hematopoietic stem cells (HSCs) to use in Sickle Cell gene therapies. It sought to enroll ten adults with SCD to determine tolerability of both the mono- and combination therapy. The study was completed in 2025 with final results to be presented at the American Society of Hematology (ASH) Annual Meeting in December 2025.
Safety
Data from the clinical trial was made available in the abstract. The study found that motixafortide alone and in combination with natalizumab were safe and well tolerated. Adverse events included Grade 1 and 2 injection-site and systemic reactions including pruritus (90%), pain or tingling (80%) and urticaria (40%). No grade 4 adverse events, dose limiting toxicities or complicated vaso-occlusive crises were observed.
Efficacy
Motixafortide alone and in combination with natalizumab generated CD34+ HSC mobilization to the peripheral blood (PB). By itself, motixafortide mobilized a median of 189 CD34+ cells/μl (ranging from 77-690) to the PB at 10-14 hours post motixafortide administration, with a median 4.22 x 106 CD34+ cells/kg as part of a single blood volume collection. Based on this performance, investigators project the collection of 16.9 x 106 HSCs in a normal, four session, single-day apheresis collection period. Motixafortide in combination with natalizumab mobilized a median of 312 CD34+ cells/μl (range 117-447) at 14 hours post motixafortide administration, with median 4.89 x 106 CD34+ cells/kg collected as part of a single blood volume collection, projecting the collection of 19.6 x 106 CD34+ HSCs in a single-day four-blood-volume apheresis collection session. In two subjects with prior plerixafor-based mobilization, motixafortide alone and in combination with natalizumab led to 2.7 to 2.8 fold higher PB CD34+ cells/μl and 2.8 to 3.2 fold higher CD34+ cells/kg, respectively. Two phenotypic SCD subgroups were identified with distinct mobilization kinetics. Four of the ten adults were “super” mobilizers that were able to mobilize about 4.6x greater CD34+ HSCs on average compared with the six standard mobilizers using motixafortide. When motixafortide was combined with natalizumab the difference in “super” vs. standard mobilizers was not significant.
Conclusion
The study concluded that motixafortide alone and in combination with natalizumab can safely mobilize HSCs in SCD patients. We think that there is a significant and growing need for agents that better mobilize HSCs for gene therapy, gene editing and ex vivo cell-manufacturing-based therapies. A therapy cannot proceed if there are insufficient HSCs and in some cases such as SCD, granulocyte colony stimulating factor (G-CSF) cannot be used without substantial risk. An agent that can help produce sufficient HSC can help one of the most important bottlenecks in SCD and more broadly in other cell-based therapies.
GILX1 Patent Notice of Allowance
On November 17th, BioLineRx announced that it has received a notice of allowance from the US Patent and Trademark Office (USPTO) for GLIX1 for treating a broad range of cancers. Specifically, the patent covers the use of GLIX1 for cancers where cytidine deaminase (CDA)[1] is not overexpressed. CDA is not overexpressed in a majority of cancers. The granted patent would provide protection until 2040 with possible patent extension for up to five years. It is entitled Deoxy-Cytidine or Uridine Derivatives for Use in Cancer Therapies and is listed under patent serial number 18/602,969. It covers the use of GLIX1 for treating cancers where CDA is not over-expressed beyond a specific threshold. Similar patents are being pursued worldwide.
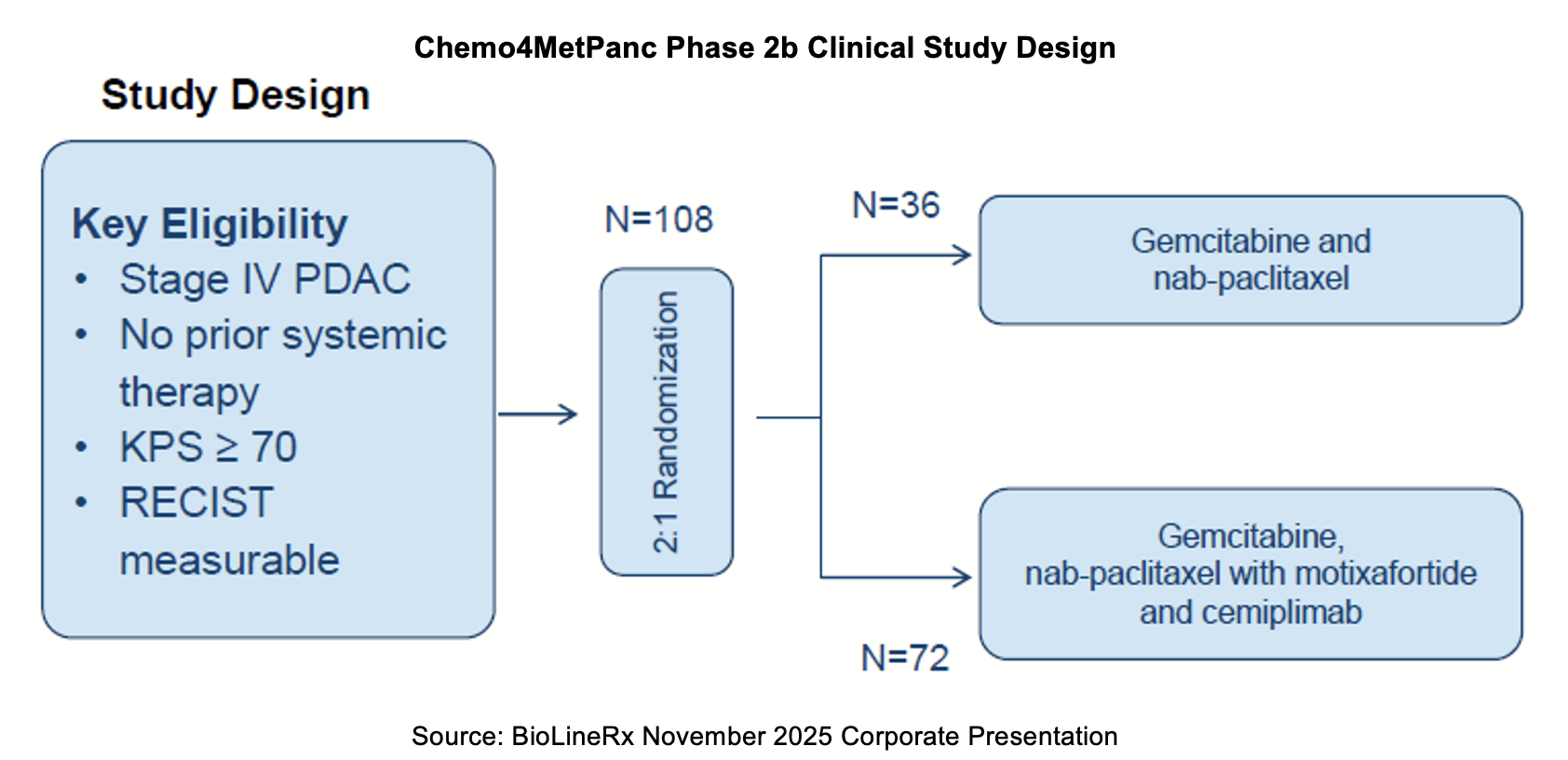
CheMo4METPANC Study
In May 2025, investigators at Columbia University reported updated results from the pilot phase of the Chemotherapy (Gemcitabine + Nab-paclitaxel), Motixafortide (CXCR4 inhibitor), and 4 (for) METastatic PANCreatic cancer (CheMo4METPANC) study. The data indicated that four of 11 patients remained progression free after more than one year. Two patients underwent definitive treatment for mPDAC. One patients’ radiologically detected liver lesions completely resolved. All patients received definitive radiation to the primary pancreatic tumor, and one exhibited a sustained partial response and underwent pancreaticoduodenectomy with pathology demonstrating a complete response. An analysis of pre- and on-treatment biopsies and peripheral blood mononuclear cells also revealed that CD8+ T-cell tumor infiltration increased across all eleven patients treated with the motixafortide combination.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
________________________
[1] Cytidine deaminase (CDA) is an enzyme that plays a key role in pyrimidine metabolism, specifically in the salvage and breakdown of cytidine-containing nucleosides. Cytidine deaminase is important in cancer treatment because of how it interacts with certain chemotherapy drugs.