By David Bautz, PhD
NASDAQ:COCP
READ THE FULL COCP RESEARCH REPORT
Business Update
CDI-988 Overview Presented at Calicivirus Conference
On September 12, 2025, Cocrystal Pharma Inc. (NASDAQ:COCP) announced that its Presidents and Co-CEO, Dr. Sam Lee, presented an overview of the company’s lead pan-viral protease inhibitor, CDI-988, at the 9th International Calicivirus Conference. A copy of the presentation can be found here. CDI-988 is being developed as a treatment for both coronavirus and norovirus infection as well as norovirus prophylaxis.
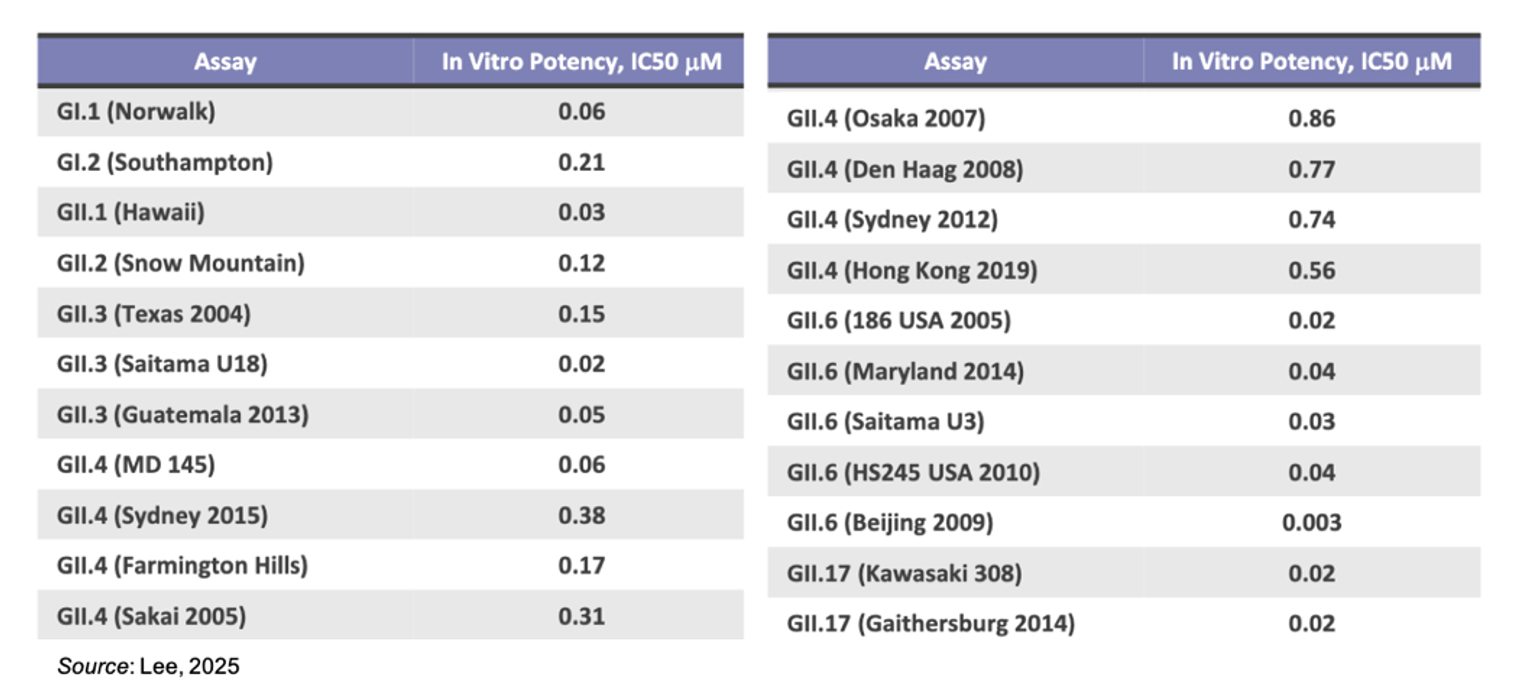
The presentation noted that there has been a rapid surge of norovirus outbreaks in the past year following the COVID-19 pandemic. This has been accompanied by a transition from strain GII.4 being the dominant strain to GII.17. Thus, in order to create an effective broad-spectrum norovirus therapy, it is necessary to have activity against multiple strains of the virus. CDI-988 shows broad-spectrum binding against multiple norovirus strains, as shown in the following results for in vitro potency assays.
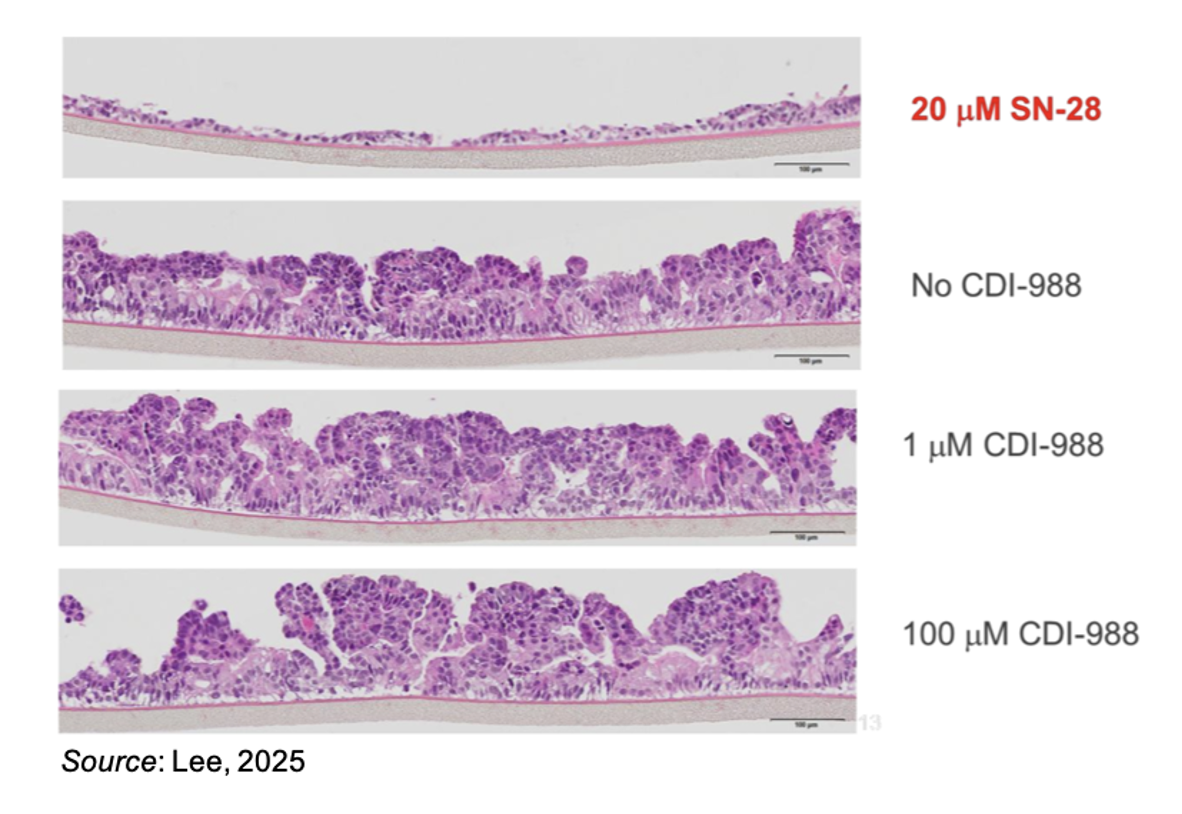
To test the safety of CDI-988, the company performed a comprehensive safety assessment using human 3-D small intestinal tissue system. This system will demonstrate if there are any potential cytotoxic effects on small intestine cells. The following image shows that compared to treatment with SN-38, a reference compound that causes GI toxicity including diarrhea, nausea, and vomiting, treatment of the cell system with CDI-988 showed no discernible effect compared to untreated cells.
Lastly, pharmacokinetic data showed that CDI-988 has significantly higher exposure and a longer half-life in the intestine and stomach compared to the plasma. Thus, the drug concentration should be highest where it is needed, in the norovirus-infected areas of the small intestine.

The remainder of the presentation focused on the Phase 1 clinical trial of CDI-988. The study enrolled 46 (N=36 drug; N=10 placebo) individuals into the single ascending dose (SAD) cohort and 48 (N=36 drug; N=12 placebo) individuals into the multiple ascending dose (MAD) cohort. The SAD results showed that all doses (100 mg to 1200 mg) were well tolerated, there were no reports of serious adverse events, no clinically relevant ECG changes, no clinically significant pathology results, and no discontinuations from the study or use of the study drug. Similar results were seen in the MAD cohort, as all doses (200 mg to 1200 mg) were well tolerated, there were no reports of serious adverse events, no clinically relevant ECG changes, and no clinically significant pathology results. There was one discontinuation from the study and study drug due to Grade 2 diarrhea for an individual in the 1200 mg BID Fed group. This discontinuation was deemed probably related to study drug. CDI-988 shows a strong food effect (5-fold higher plasma exposure when administered after a high-fat meal), thus that may have contributed to the Grade 2 diarrhea for that individual.
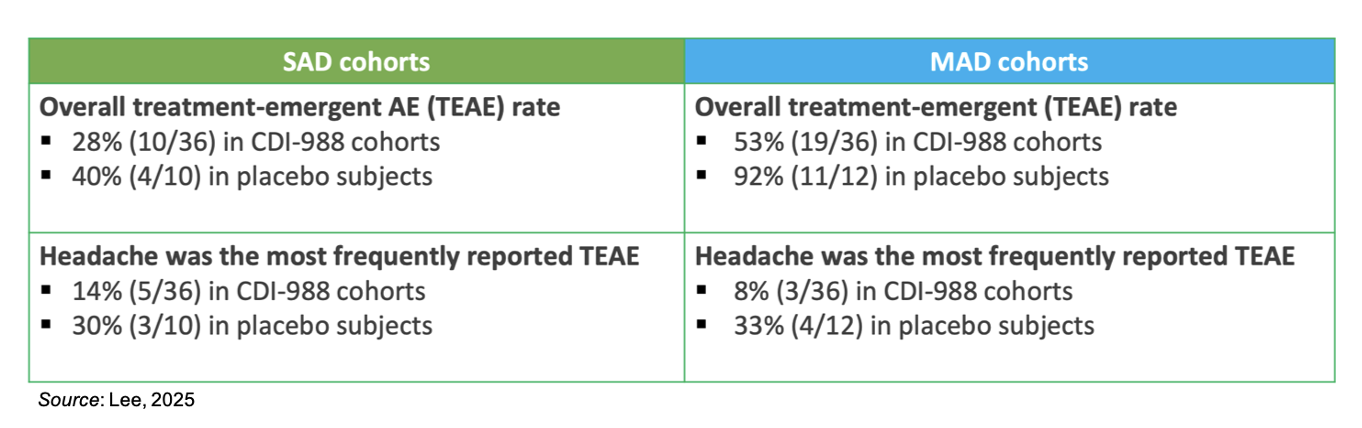
The topline safety data are summarized in the table below. Headache was the most frequently reported treatment emergent adverse event (TEAE) and overall the placebo groups had a higher frequency of TEAEs than the CDI-988 groups.
The next step for this program is a Phase 1b human challenge study. The company recently announced that the FDA issued a Study May Proceed Letter for that trial, which will utilize the GII.2 norovirus strain according to a published protocol (Rouphael et al., 2022). We anticipate the trial initiating before the end of 2025.
Financial Update
On September 15, 2025, Cocrystal announced the closing of up to a $13 million registered direct offering that consisted of approximately 2.76 million shares of common stock at a purchase price of $1.70 for gross proceeds of approximately $4.7 million and the issuance of unregistered warrants to purchase up to approximately 5.53 million shares of common stock at an exercise price of $1.50, that could result in an additional approximately $8.3 million in gross proceeds if the warrants are fully exercised on a cash basis.
Conclusion
The data compiled thus far for CDI-988 has been very encouraging and we look forward to the initiation of the Phase 1b norovirus challenge study, which we anticipate beginning before the end of 2025. In addition, we anticipate an update later this year on CC-42344, which is currently in a Phase 2a clinical trial for the treatment of influenza. We had previously accounted for an expected financing in our model and our valuation remains at $8 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.