By David Bautz, PhD
NASDAQ:EDSA
READ THE FULL EDSA RESEARCH REPORT
Business Update
Gearing Up for Phase 2 Vitiligo Trial in Mid-2026
Edesa Biotech, Inc. (NASDAQ:EDSA) is planning for a Phase 2 study of EB06, its anti-CXCL10 monoclonal antibody, for the treatment of moderate-to-severe non-segmental vitiligo patients. Vitiligo is a disease that causes areas of the skin to lose color, with non-segmental vitiligo being characterized by patches appearing on both sides of the body. It is caused when pigment-producing cells (melanocytes) die or stop producing melanin as a result of an autoimmune disease, genetics, or a triggering event (e.g., stress, sunburn, skin trauma).
Past research showed that the chemokine CXCL10 was elevated in both vitiligo patient skin and serum (El-Domyati et al., 2022). In a mouse model of vitiligo, which includes CXCL10 expression in the skin, neutralization of CXCL10 in mice with established, widespread depigmentation induced reversal of disease as shown by repigmentation (Rashighi et al., 2014). In addition, serum CXCL10 levels are significantly increased in vitiligo patients compared to controls, suggesting that CXCL10 may play a role in the pathogenesis of vitiligo in humans (Gharib et al., 2021). The following slide gives an overview of the mechanism of action of EB06 and data supporting its use in the treatment of vitiligo.
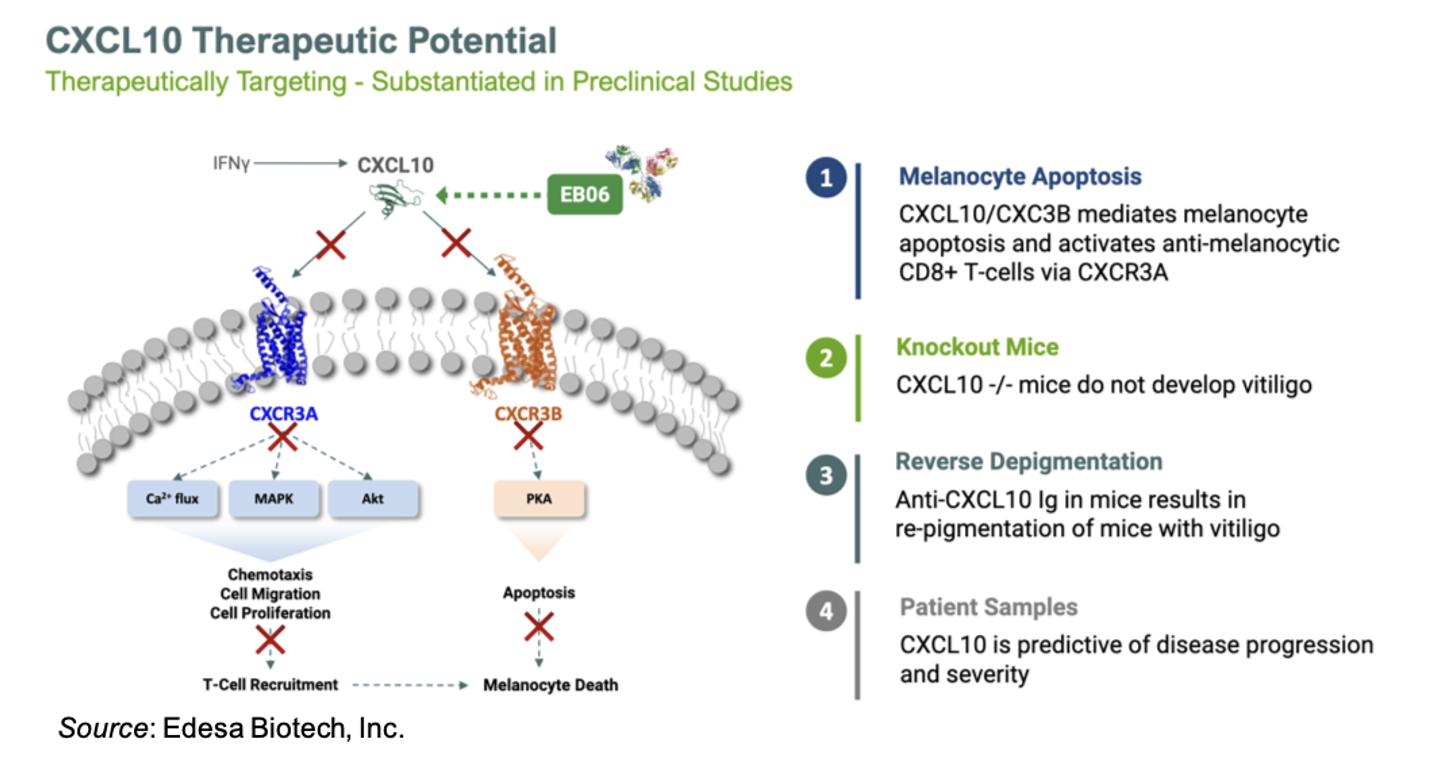
A 2022 publication reported that the estimated prevalence of vitiligo patients in the U.S. is between 1.9 million and 2.8 million (Gandhi et al., 2022). This corresponds to a vitiligo market that is projected to reach approximately $1.1 billion by 2030 (EvaluatePharma). Currently, the only FDA approved therapy is topical ruxolitinib (Opzelura®), which generated approximately $500 million in revenue in 2024, with approximately $200 million of that coming from sales for vitiligo (EvaluatePharma). Opzelura carries a black-box warning due to the potential for serious infections, major adverse cardiovascular events, and thrombosis (Opzelura prescribing information). Thus, there is clearly an unmet need for additional safe and effective treatment options for vitiligo patients.
Edesa is currently readying an IND submission for EB06 and has already received approval from Health Canada to conduct a Phase 2 trial. In addition, the company has initiated manufacturing activities to supply drug product for the Phase 2 trial. Edesa has also begun outreach to potential investigators. The study as currently planned will enroll approximately 160 patients with severe nonsegmental vitiligo, will evaluate three different doses of EB06 (2.5 mg/kg, 5 mg/kg, 10 mg/kg) administered IV every two weeks for up to 24 weeks followed by a 12-week follow up period, and will have a primary efficacy outcome of the percentage of patients that achieve ≥50% decrease from baseline in facial Vitiligo Area Scoring Index (F-VASI50), a composite measurement of the overall area of facial vitiligo patches and degree of depigmentation within patches. The final trial protocol will be contingent on feedback from the FDA, and we anticipate enrollment initiating in mid-2026, dependent upon completion of manufacturing and regulatory activities.
Positive Phase 3 Results for EB05 in ARDS
In October 2025, Edesa announced positive results from the Phase 3 trial evaluating paridiprubart (EB05) in the treatment of acute respiratory distress syndrome (ARDS). ARDS is caused by an exaggerated immune response that leads to an unregulated inflammatory response in the body and ultimately injury to the lungs that results in oxygen deprivation. There are a number of different causes, including virus-induced pneumonia, smoke/chemical inhalation, sepsis, and chest injury. There are approximately 600,000 ARDS-related ICU admissions every year across the seven major markets (U.S., U.K., Germany, France, Spain, Italy, Japan, and Canada). There are currently few treatment options for moderate to severe ARDS aside from supplemental oxygen and mechanical ventilation. ARDS imposes a very high burden on the healthcare system, both in terms of cost (averages >$100,000 per patient in the U.S.) and a very high mortality rate.
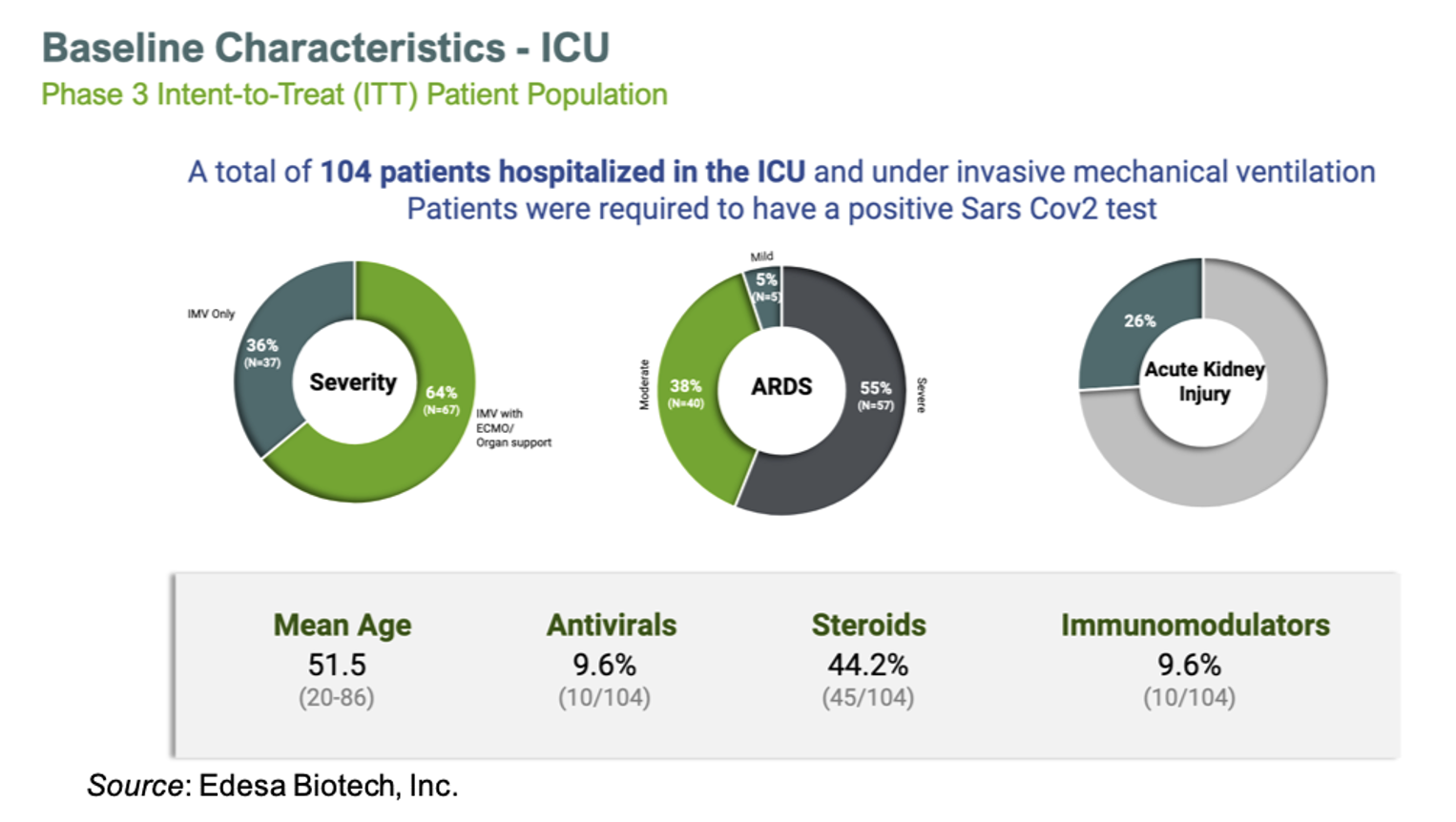
The Phase 3 trial of EB05 enrolled a total of 104 patients that were hospitalized in the ICU, receiving invasive mechanical ventilation (IMV), and had a positive SARS-CoV-2 test. The baseline characteristics of the intent-to-treat population is given below. The majority of patients were receiving IMV with ECMO/organ support, had severe ARDS, and over a quarter of them had acute kidney injury.
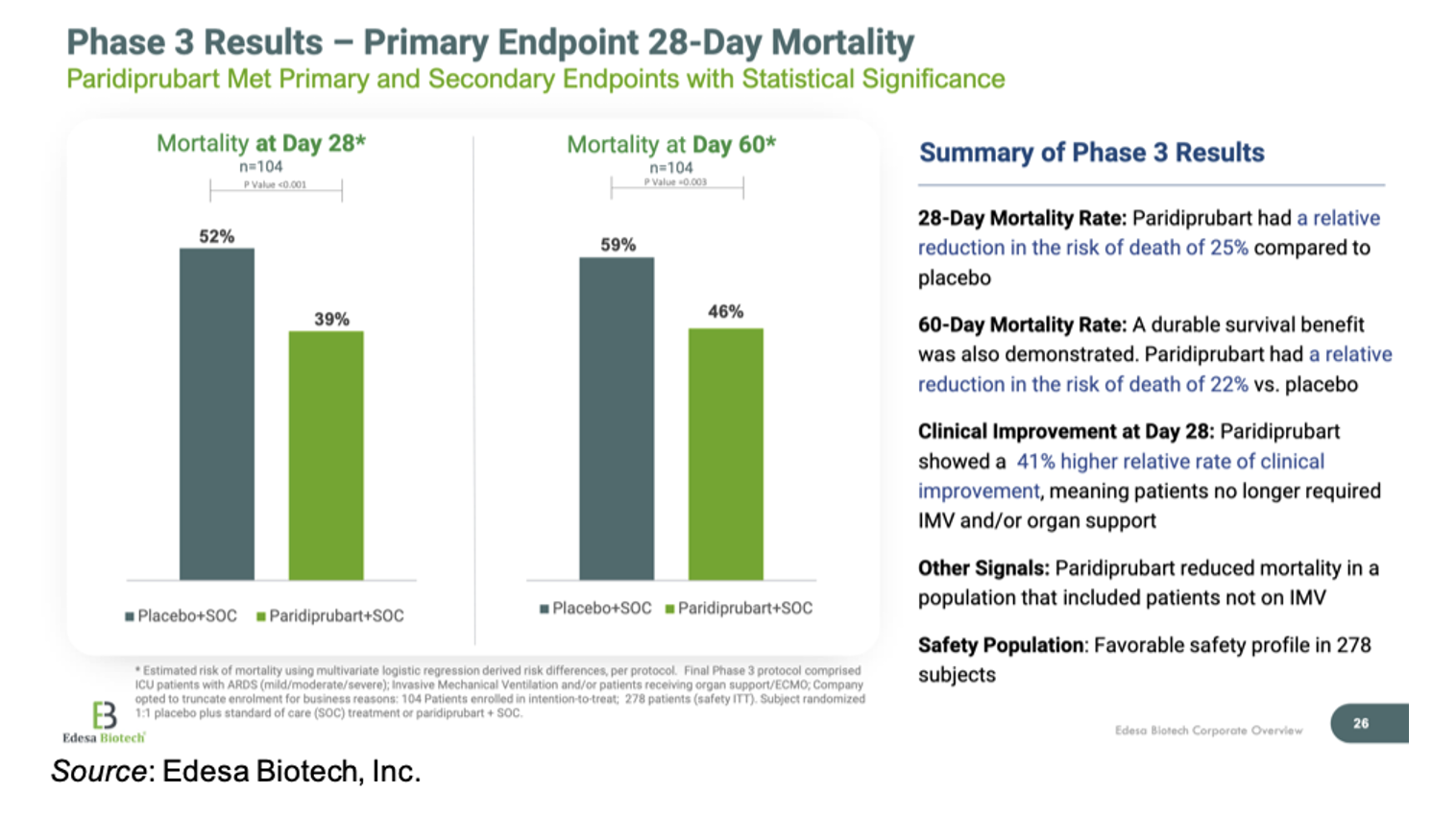
The study met the primary endpoint by showing a statistically significant improvement in 28-day mortality for patients treated with standard of care (SOC) + EB05 compared to those receiving only SOC (P<0.001). The relative reduction in the risk of death at Day 28 was 25% for EB05-treated patients compared to placebo. Importantly, a durable survival benefit was shown as the relative reduction in the risk of death at Day 60 was 22% for EB05-treated patients compared to placebo. The results also show just how sick of a population the Phase 3 study enrolled, with over half of placebo-treated patients dying by Day 28. This underscores how important these results are for a patient population that has few effective treatment options.
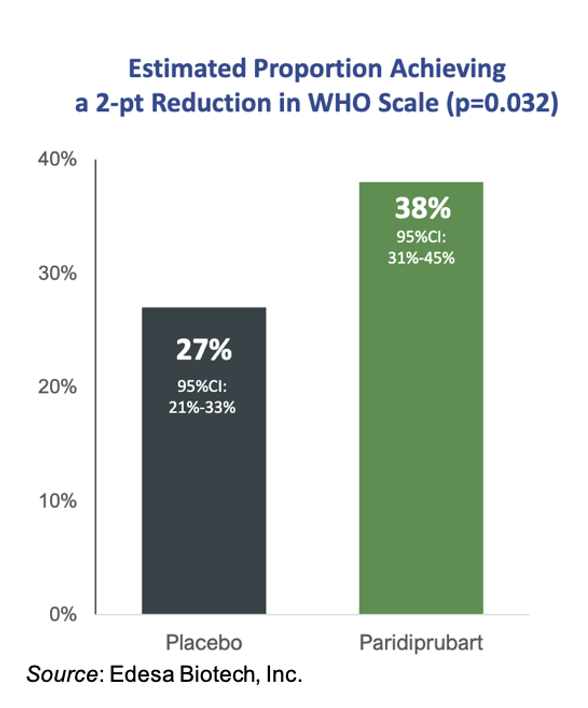
In addition to improving mortality, treatment with EB05 also led to a significant improvement in clinical improvement, which was defined as at least a 2-point reduction in the WHO 9-point ordinal scale (WHO). The following graph shows that 38% of EB05-treated patients had a ≥2-point reduction in the WHO scale compared to only 27% receiving SOC (P=0.032). These results indicate that not only is treatment with EB05 preventing patients from dying, but it is also helping to improve patients’ quality of life, for example, moving them out of the ICU and off of mechanical ventilation.
Lastly, EB05 has exhibited a very favorable safety profile. The following table shows the safety outcomes for the 278 patients in the safety database (n=138 EB05; n=140 placebo). There were no treatment-related adverse events observed, and the event profile between the two treatment groups was very similar. EB05 has been dosed in over 460 patients and healthy volunteers through its development, which supports its favorable safety profile.
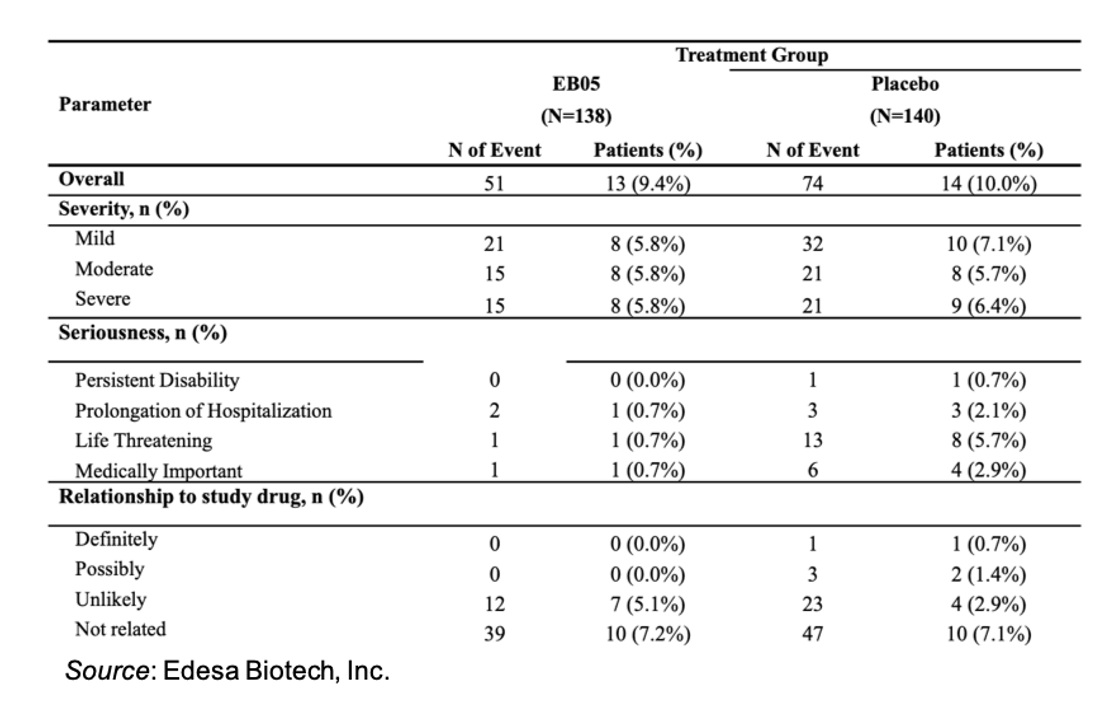
EB05 is also currently being evaluated in a $117M platform study funded by BARDA that is evaluating three novel therapeutics for ARDS (no necessity for a SARS-CoV-2 positive test). The company is supplying EB05 through manufacturing scale-up that is supported by the Government of Canada’s Strategic Innovation Fund, thus no company resources are being utilized for that study.
Financial Update
On December 12, 2025, Edesa announced financial results for fiscal year 2025 that ended September 30, 2025. As expected, there were no revenues reported for fiscal year 2025. R&D expenses in fiscal year 2025 were $3.7 million, compared to $2.9 million for fiscal year 2024. The increase was primarily due to increased expenses for manufacturing-related activities and other preparations for a planned Phase 2 clinical study of EB06 in vitiligo, along with increased expenses related to the completion of the Phase 3 study of EB05, partially offset by lower spending on other development programs. G&A expenses totaled $4.2 million in fiscal year 2025 compared to $4.1 million for fiscal year 2024. The increase was primarily due to an increase in non-cash, share-based compensation partially offset by a decrease in professional fees.
As of September 30, 2025, Edesa had approximately $10.8 million in cash and cash equivalents. Subsequent to the end of the year, Edesa received $3.4 million in net proceeds from the sale of stock under its at-the-market offering program. As of December 12, 2025, the company had approximately 8.3 million common shares outstanding and, when factoring in stock options, warrants, and restricted stock units, a fully diluted share count of approximately 15.2 million.
Conclusion
We are glad to see that the company remains on track to initiate a Phase 2 trial in vitiligo in 2026. We currently estimate enrollment will begin in the middle of the year, contingent upon the company completing manufacturing and regulatory activities. With only one FDA approved treatment, which carries a black box warning, there is ample opportunity for a safe and effective vitiligo treatment in a market that is projected to be over $1 billion in 2030. With no changes to our model, our valuation remains at $19 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.