By David Bautz, PhD
NASDAQ:MTVA
READ THE FULL MTVA RESEARCH REPORT
Business Update
DA-1241 in Combination with Efruxifermin Shows Enhanced Hepatoprotective Effects
On June 21, 2025, MetaVia Inc. (NASDAQ:MTVA) announced the presentation of preclinical data for DA-1241, the company’s novel G-protein Coupled Receptor 119 (GPR119) agonist, in combination with efruxifermin, a fibroblast growth factor 21 (FGF21) analogue, in a metabolic dysfunction-associated steatohepatitis (MASH) mouse model. The data was presented in a poster session at the 85th American Diabetes Association Scientific Sessions. A copy of the presentation can be found here.
The study utilized mice fed the Gubra Amylin NASH (GAN) diet for 36 weeks. The GAN diet induces metabolic and histopathologic hallmarks of fibrotic NASH (Veidal et al., 2019). Mice were then randomized to receive either placebo, DA-1241 (100 mg/kg once daily, oral), efruxifermin (EFX, 1mg/kg once weekly, subcutaneous), or the combination therapy for 12 weeks. An outline of the study is given below.
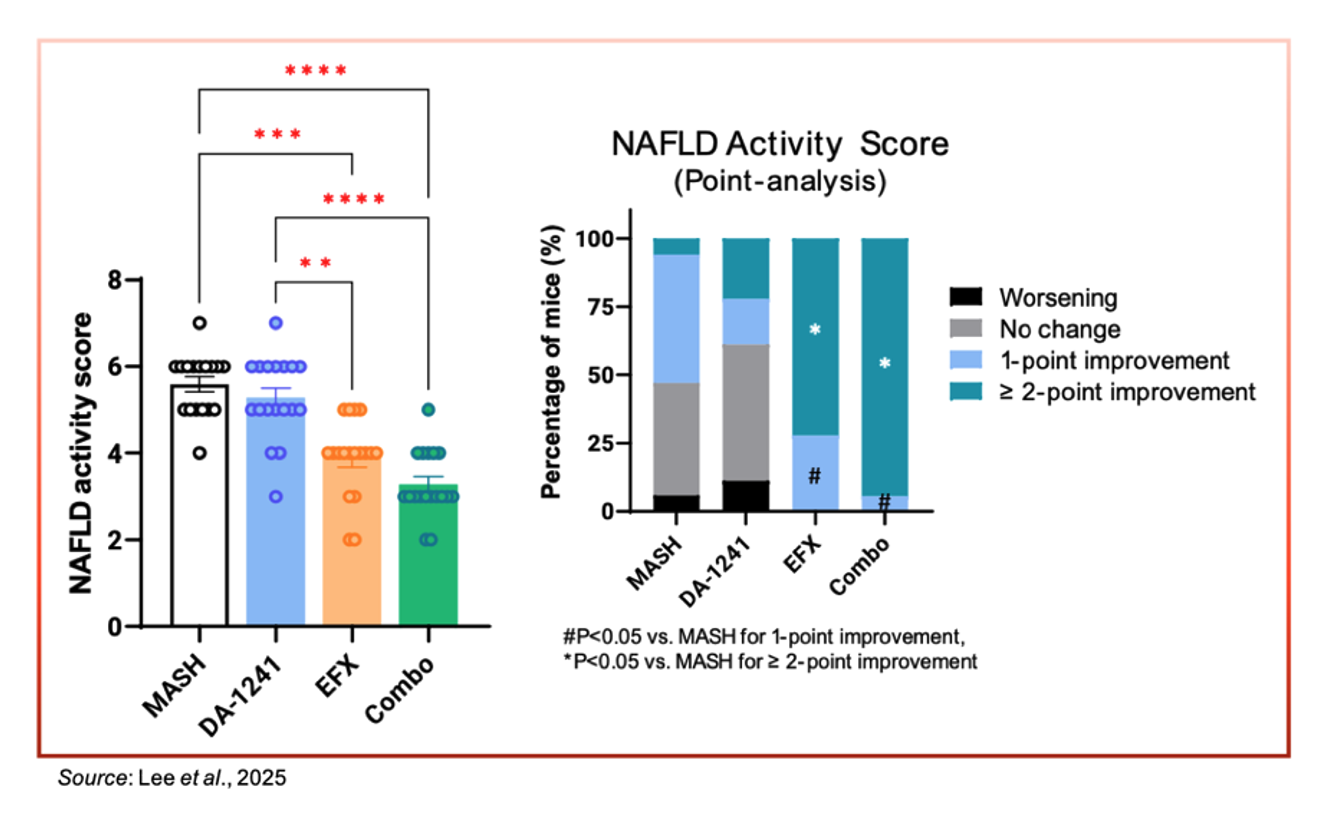
The following image shows the change in NAFLD Activity Score (NAS) following 12 weeks of treatment. The combination therapy showed a significant improvement in NAS compared to the MASH control group. In addition, enhanced activity was noted in the control group compared to either monotherapy cohort.
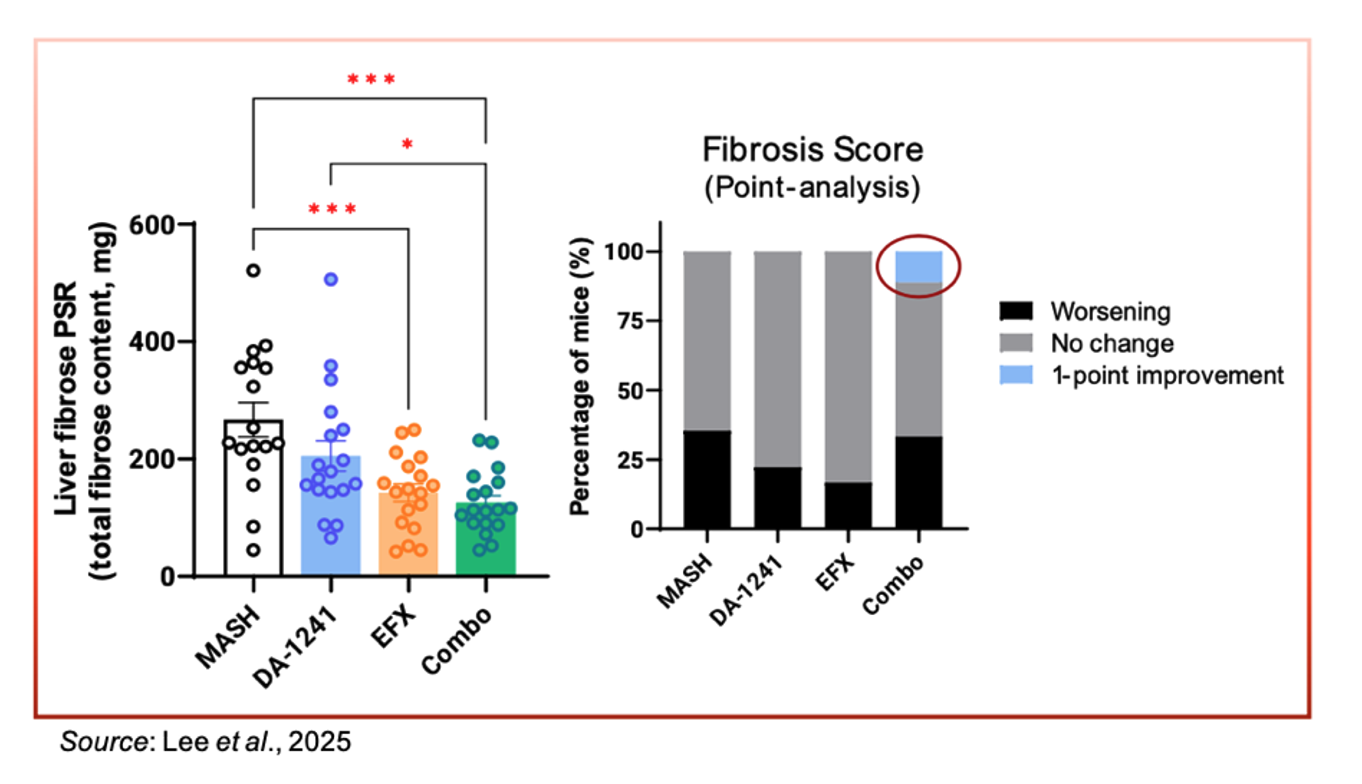
In regards to change in fibrosis, the combination of DA-1241 and EFX resulted in a significant reduction in liver fibrosis, and a subset of animals in that cohort experienced an improvement in the fibrosis score. No improvement in fibrosis score was seen in the monotherapy groups.
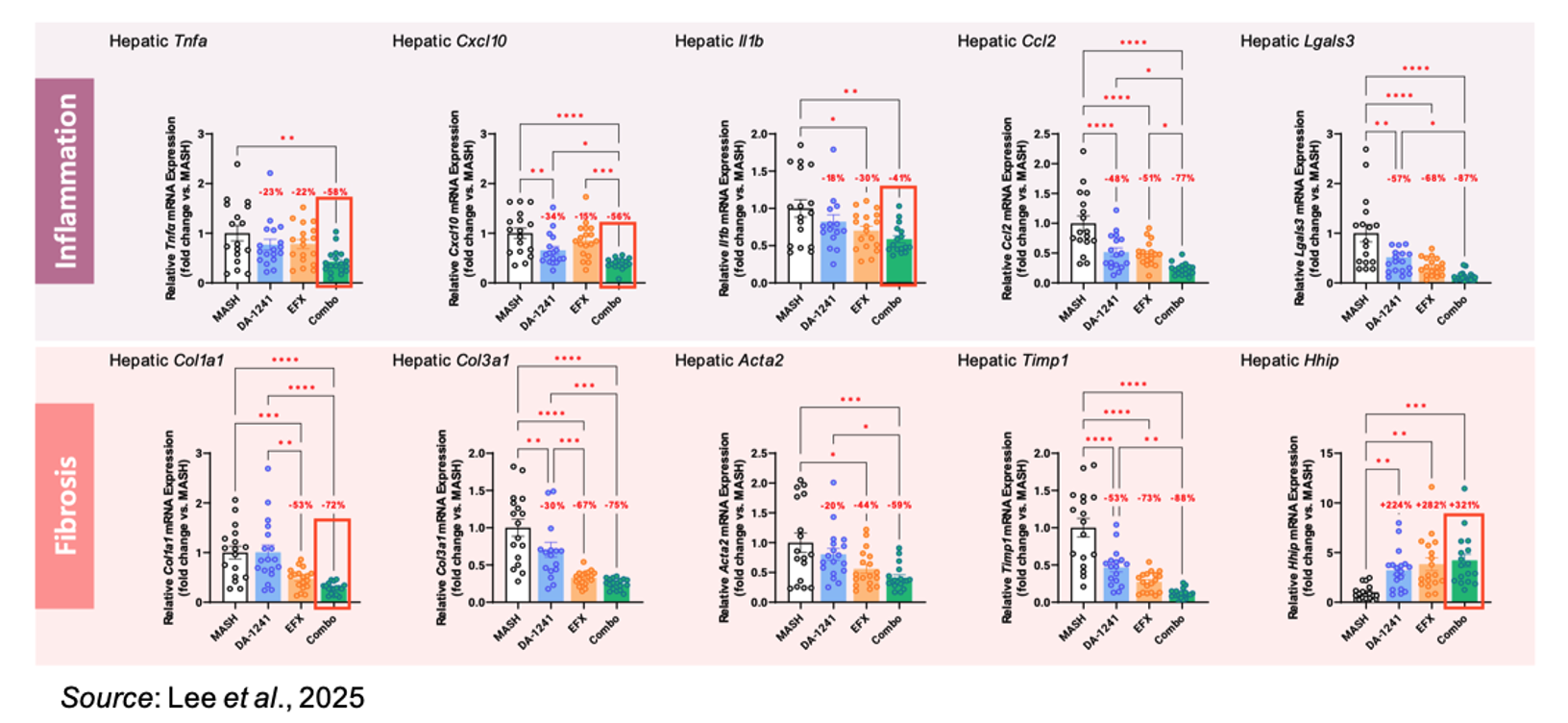
An analysis of various key liver genes associated with inflammation and fibrosis revealed a significant reduction in the expression of Tnfa, Cxcl10, Il1b, and Col1a while a significant increase was noted in Hhip, which is a suppressor of hepatic stellate cell activation. In addition to a decrease in expression of TNF-α in the liver, there was also a significant decrease in plasma TNF-α levels in the combination group, suggesting a positive effect on systemic inflammation.
In summary, DA-1241 in combination with EFX resulted in an improvement in plasma ALT, liver cholesterol, steatosis, inflammation, and fibrosis more than either monotherapy. In addition, 94% of mice treated with the combination therapy achieved a ≥2-point NAS improvement compared to baseline. The combination therapy also reduced a number of different inflammatory markers in the liver and plasma. These results support the therapeutic potential of DA-1241 with an FGF21 analogue for the treatment of MASH.
Conclusion
MetaVia has consistently taken the position that DA-1241 would be best suited as part of a combination therapy for the treatment of MASH. Previously, the company reported Phase 2a data that showed DA-1241 monotherapy was more effective than in combination with the DPP-4 inhibitor sitagliptin. While that combination did not appear to be effective, the use of DA-1241 with an FGF21 analogue does appear quite promising in a preclinical MASH model. We expect the company will investigate additional DA-1241 combination therapies while pursuing a partnership, collaboration, and/or licensing deal to advance DA-1241 in clinical trials. With no changes to our model, our valuation remains at $21 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.