By David Bautz, PhD
NASDAQ:MTVA
READ THE FULL MTVA RESEARCH REPORT
Business Update
New Data from Phase 2a Trial of Vanoglipel (DA-1241) Presented at The Liver Meeting 2025
In November 2025, MetaVia, Inc. (NASDAQ:MTVA) announced new data from the Phase 2a clinical trial of vanoglipel (DA-1241) was presented in a poster session at the American Association for the Study of Liver Diseases (AASLD) The Liver Meeting® 2025. A copy of the poster can be found here.
Vanoglipel is a potent and selective agonist for G protein-coupled receptor 119 (GPR119). GPR119 is a G protein-coupled receptor (GPCR) that is predominantly expressed in the pancreas, liver, and gastrointestinal tract. It is activated by a large number of naturally occurring lipid molecules and elicits physiological responses through binding to G proteins that activate adenylate cyclase and cyclic AMP signaling (Hassing et al., 2016). GPR119 activation leads to glucose-dependent insulin release from the pancreas and intestinal secretion of incretins, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) (Chu et al., 2008), while also suppressing food intake to reduce body weight gain (Overton et al., 2006).
The Phase 2 study enrolled a total of 109 subjects with presumed metabolic dysfunction-associated steatohepatitis (MASH) and qualifying baseline alanine aminotransferase (ALT). Subjects were randomized to receive placebo, vanoglipel 50 mg, vanoglipel 100 mg alone, or vanoglipel 100 mg with a DPP4 inhibitor in a 2:1:2:2 ration once daily for 16 weeks (NCT06054815).
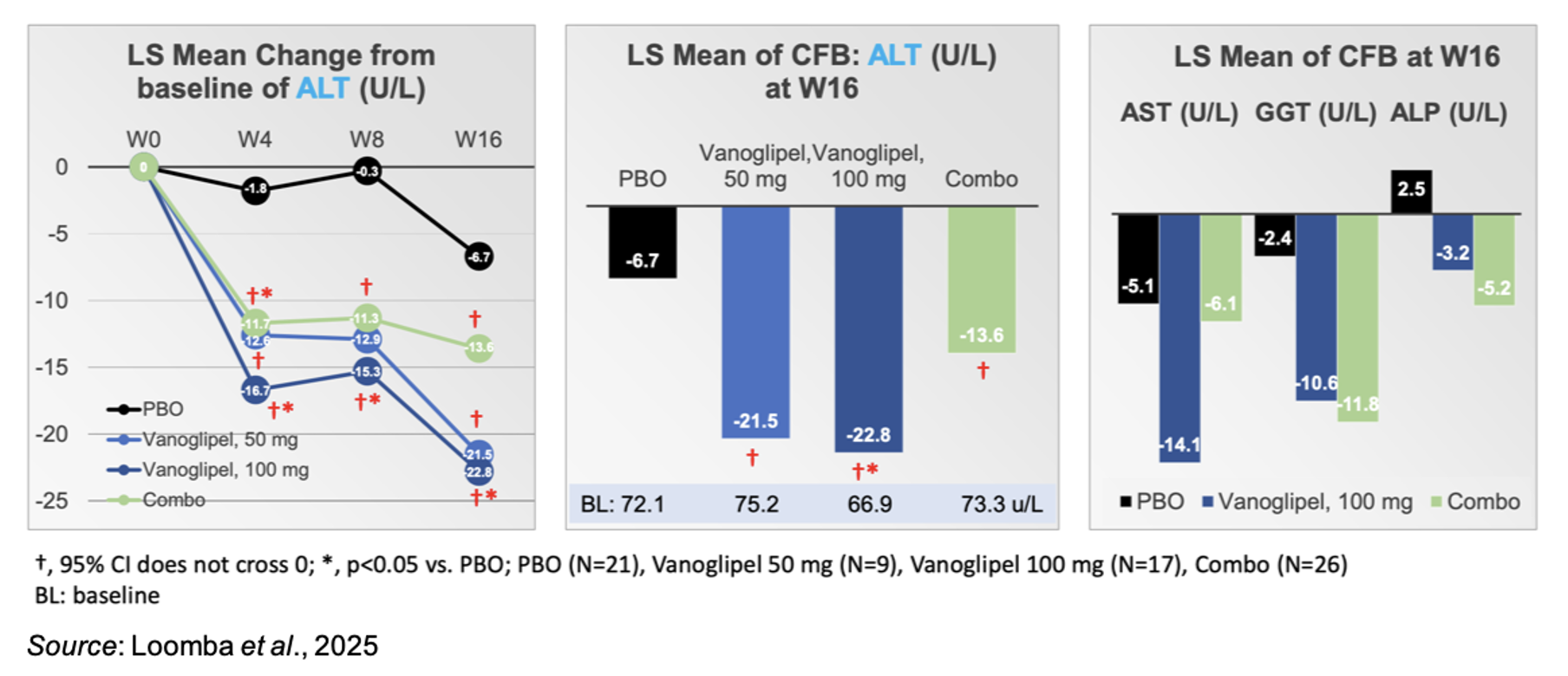
Following 16 weeks of treatment in a subgroup of subjects with baseline ALT levels between 40 and 200 U/L, vanoglipel significantly decreased plasma ALT, as shown in the following figure. The decrease in ALT was not enhanced in the combination group.
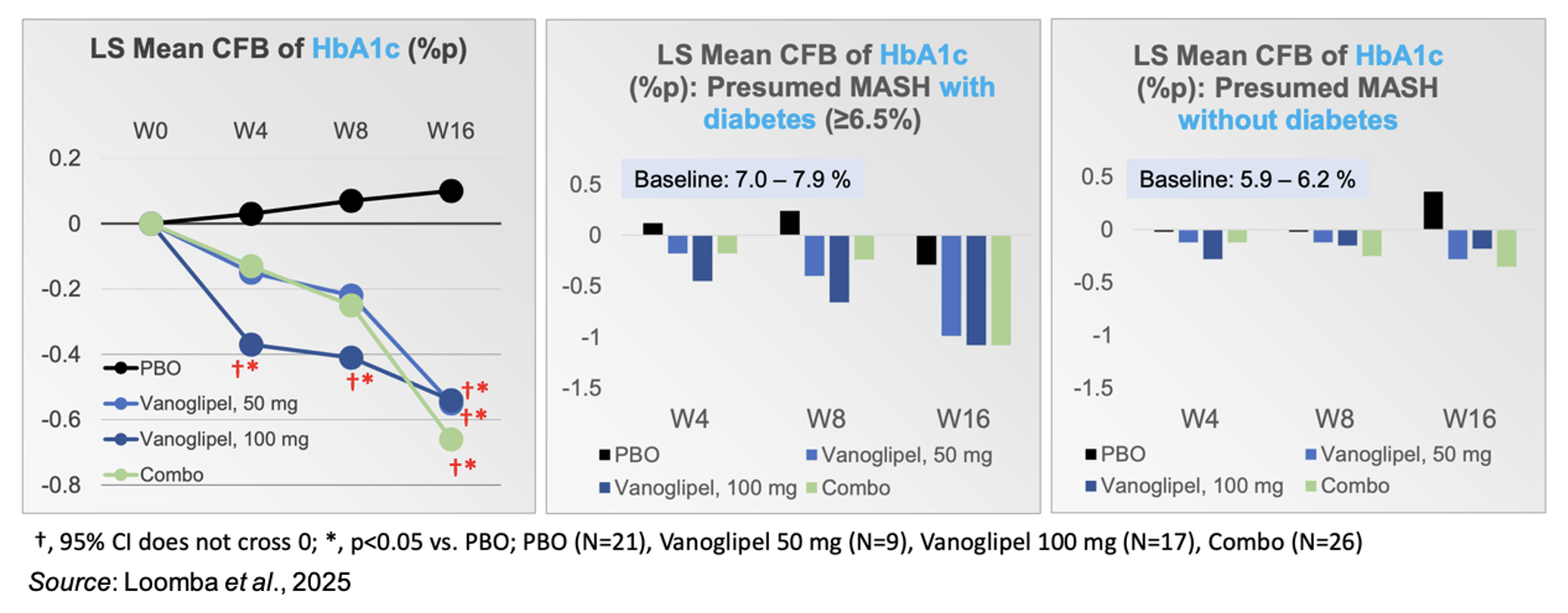
Hyperglycemia and insulin resistance in MAFLD patients can lead to oxidative stress in hepatocytes, mitochondrial dysfunction, and ultimately liver cell apoptosis (Yin et al., 2015). The results from the Phase 2a study showed that vanoglipel improved glucose control in patients with presumed MASH. The following figure on the left shows vanoglipel as a monotherapy and in combination therapy decreased HbA1c (-0.54% for monotherapy; -0.66% for combination therapy) at Week 16. The figures in the middle and the right show that vanoglipel effectively controlled plasma glucose in both prediabetic and diabetic patients. There was no difference in subjects’ weight during the study, thus highlighting the differentiated mechanism of glucose control that is independent of weight loss.
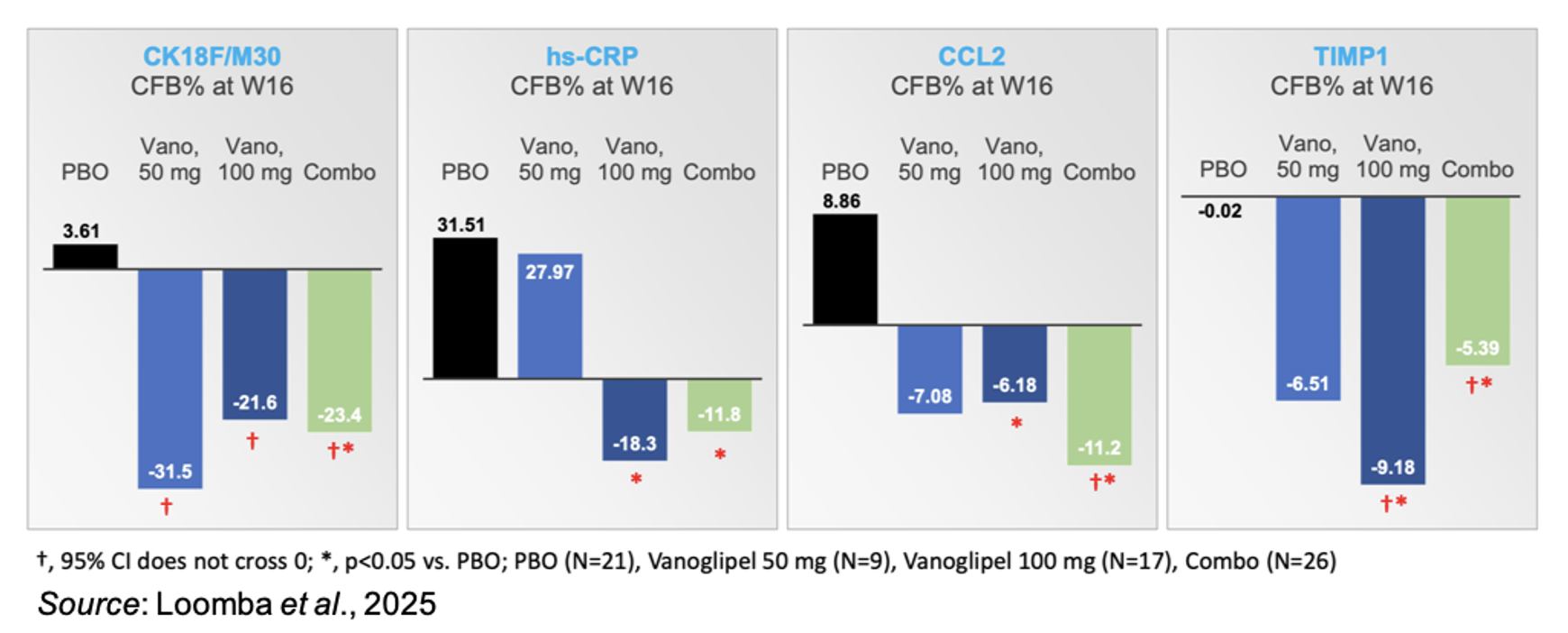
Hepatic steatosis can lead to an increase in the release of various pro-inflammatory factors (Szabo et al., 2012). Treatment with vanoglipel decreased circulating biomarkers of cell death (CK18F/M30), inflammation (hs-CRP, CCL2), and fibrosis (TIMP1). While no clear dose dependent trend was noted, most likely due to the low numbers of patient samples available, 100 mg vanoglipel consistently decreased those markers in comparison to placebo.
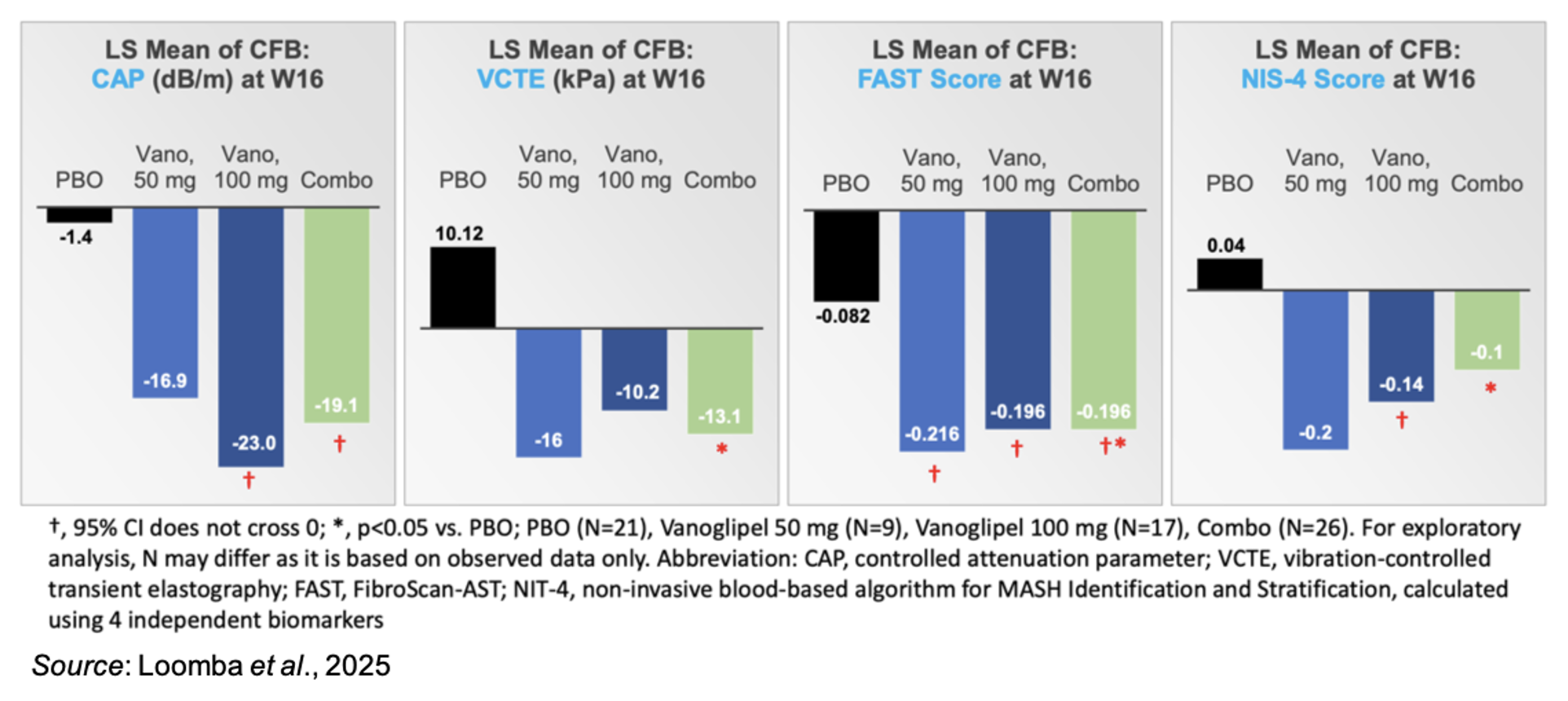
MASH patients typically have reduced glycolysis and increased triglyceride (TG) levels in the liver. TG accumulation in the liver leads to a degenerative cycle of liver cell degradation, increased inflammation, and insulin resistance. Vanoglipel improved liver steatosis as measured by controlled attenuation parameter (CAP) while also reducing liver stiffness as assessed by vibration-controlled transient elastography (VCTE) (lower left figures). Two additional non-invasive assessments were made (FAST, NIS-4), and both showed improvement from baseline (lower right figures).
In summary, vanoglipel was well tolerated with no treatment emergent adverse events leading to a treatment discontinuation except for one in the placebo group. The drug showed positive effects on both hepatic and metabolic components of MASH, including a reduction in ALT and improved glucose control. The company will be scheduling an ‘End-of-Phase 2’ meeting with the FDA in order to determine next steps for that program.
Financial Update
On December 2, 2025, MetaVia announced a 1-for-11 reverse stock split that became effective on December 4, 2025. The number of outstanding shares was reduced from approximately 25.4 million to approximately 2.3 million. We have adjusted our model and valuation to reflect the stock split.
Conclusion
The Phase 2a data for vanoglipel is very encouraging and show that, as a monotherapy, the drug is effective in treating different aspects of MASH, including both hepatic and metabolic factors. We anticipate the company providing an update on the program following an ‘End-of-Phase-2’ meeting with the FDA, but believe at this point the drug with either be out-licensed, or a partnership will be entered into before advancing it further in clinical development.
We remind investors that we continue to anticipate 48 mg 8-week data from the Phase 1 trial of DA-1726, the company’s oxyntomodulin (OXM) analog that is a dual agonist of the glucagon-like peptide-1 receptor (GLP1R) and glucagon receptor (GCGR), before the end of the year. These results should include efficacy and adverse event outcomes. We have adjusted our model to account for the reverse split, and our valuation is now $90 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.