By David Bautz, PhD
NYSE:NNVC
READ THE FULL NNVC RESEARCH REPORT
Business Update
Mpox Trial to Initiate in Late 2025/Early 2026
NanoViricides, Inc. (NYSE:NNVC) is planning to initiate a Phase 2 clinical trial for the treatment of Mpox near the end of 2025 or early 2026 for its lead development candidate NV-387. The trial will take place in the Democratic Republic of Congo (DRC), a country that has reported the greatest number of cases during the most recent Mpox outbreak (WHO). NanoViricides has obtained a preliminary approval from the Ethics Committee of the regulatory agency in charge of the African region (ACOREP) and has partnered with a Contract Research Organization (CRO) to design and conduct the Phase 2 trial. A Clinical Trial Application (CTA) is currently being developed by NanoViricides, and we anticipate that being filed in the fourth quarter of 2025.
The planned Phase 2 trial is expected to enroll 80 hospitalized MPox patients and will consist of two parts that will compare the standard of care (SOC) to NV-387 + SOC. The Phase 2a portion of the study will evaluate a fixed dose of NV-387 administered to MPox patients for six days, at which time the patients will be evaluated to determine the safety and tolerability of the drug and whether dosing should continue for longer. Ten patients will be treated with SOC, and ten will be treated with NV-387 + SOC. The Phase 2b portion of the study will compare SOC to NV-387 + SOC in MPox patients randomized 1:2 to each cohort using the dosing schedule determined in the Phase 2a portion. A total of 60 patients will be evaluated in the Phase 2b portion of the trial. The efficacy outcomes of the study are complete lesion recovery, elimination of new rash formation, viral load, and 28-day outcome. We currently estimate that the Phase 2 trial will take approximately 3-6 months to complete; however, that will be contingent upon patient recruitment and the course of the MPox outbreak.
Positive data from the MPox trial would also support the potential use of NV-387 as a smallpox therapy, which is from the same viral family as MPox (orthopoxvirus). Developing a treatment for smallpox is accomplished via the FDA’s ‘Animal Rule’, which requires a safety trial in healthy volunteers and an efficacy trial in two different animal models. The Biomedical Advanced Research and Development Authority (BARDA) is charged with the mission of developing medical countermeasures that address public health and medical consequences of chemical, biological, radiological, and nuclear (CBRN) accidents, and NanoViricides would likely partner with the agency to support the development of NV-387 for the treatment of smallpox. This could provide a valuable source of non-dilutive funding, as BARDA has supported the development of multiple other material threat medical countermeasures through grant and contract funding.
NanoViricides is also planning to apply for Orphan Drug Designation (ODD) for NV-387 for the treatment of MPox, smallpox, and measles. ODD confers a number of advantages, including the waiver of certain PDUFA fees, tax credits for research and development, increased engagement with the FDA, along with seven years of post-approval market exclusivity.
‘Empiric Therapy’ Trial Planned
In addition to the planned Phase 2 trial of NV-387 in MPox, NanoViricides is also planning to conduct a Phase 2 clinical trial of NV-387 to evaluate its potential as an ‘empiric therapy’ against a broad range of respiratory viral infections. In contrast to how antiviral medications are currently prescribed (e.g., ‘one-drug-one-bug’), given its unique mechanism of action, NV-387 has the potential to target multiple types of respiratory viruses and could potentially be utilized when patients present with a viral respiratory infection prior to identifying the specific causative agent. This is similar to how antibiotics are prescribed for bacterial infections.
In order to evaluate the potential for NV-387 as a front-line, antiviral therapy, NanoViricides is designing a ‘basket-type’, adaptive clinical trial for Viral Acute or Severe Acute Respiratory Infections (Viral-ARI or Viral-SARI). By performing an ‘all-comers’ respiratory virus trial, the company is hoping to obtain clinical efficacy data for NV-387 against multiple viruses, including influenza, RSV, coronaviruses, human metapneumovirus (hMPV), adenoviruses, echoviruses, picornaviruses, and rhinoviruses. Positive results would support the advancement of NV-387 into Phase 3 clinical testing against those viruses that the drug showed activity against.
The company has previously tested NV-387 in multiple viral animal models, including an RSV model, an Influenza model, and an Ectromelia model. The results of these studies are summarized below.
RSV
The activity of NV-387 as both an oral treatment and injectable was tested in mice lethally challenged with RSV A2 virus. Ribavirin, the only drug currently approved to treat RSV infection, was utilized as a positive control. The results showed that both oral and injected NV-387 came close to matching the efficacy of ribavirin in extending survival of lethally infected mice compared to those treated with placebo.
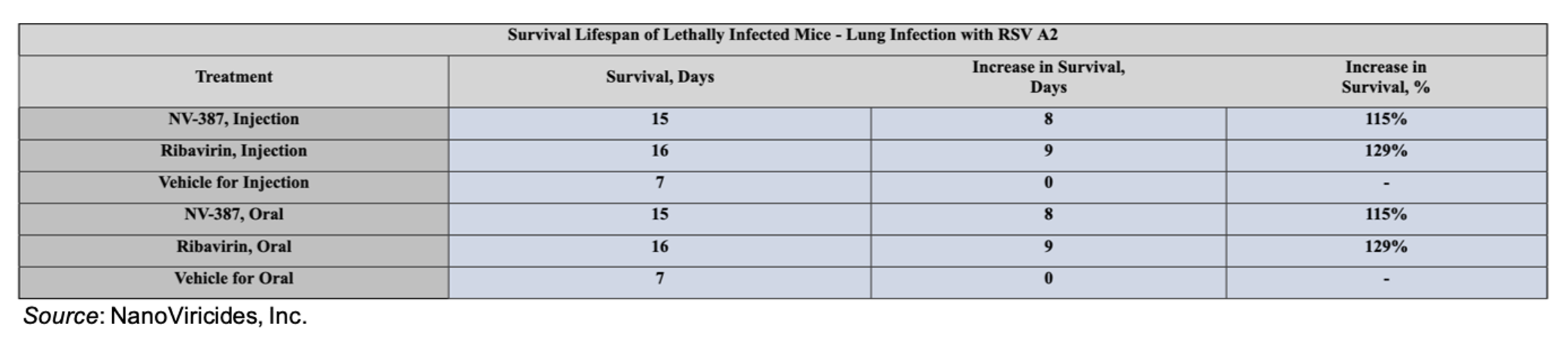
In a second RSV experiment, oral dosing of NV-387 was extended to ten days, with two doses on the first day, along with increased dosing for ribavirin as well. The results of this study showed that NV-387 completely protected the mice from dying and prevented the formation of lung damage in the animals.
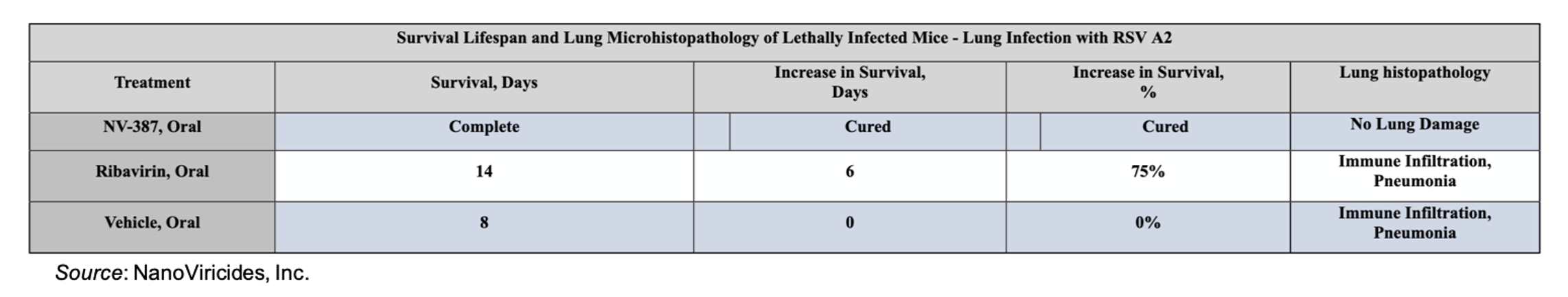
The results from these two studies show that NV-387 compares quite favorably to ribavirin for the treatment of RSV, which is even more important given the known toxicity and side effect profile of ribavirin (which includes the potential for hematological and nephrological adverse events).
Influenza
NV-387 was tested in an influenza infection model against Influenza A/H3N2. NV-387 was dosed twice on day one and once daily for an additional eight days. It was tested along with oseltamivir (Tamiflu®), dosed orally twice daily for eight days, baloxavir (Xofluza®), given orally as a single dose, and peramivir (Rapivab®), dosed by tail-vein injection once daily for eight days. The results of the study are shown below. Compared to placebo-treated mice, NV-387-treated mice lived longer than mice treated with any of the other therapies.
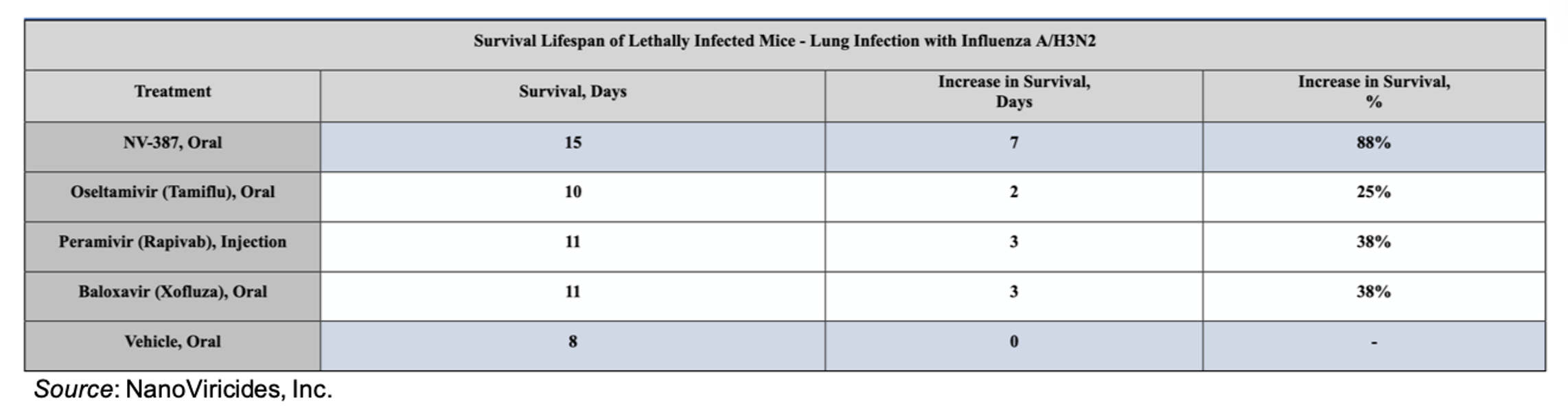
In addition, NV-387 treatment reduced the lung mucus index (53 vs. 138 for untreated animals), which measures lung congestion and is related to pneumonia symptoms, and also decreased the presence of infiltrating immune cells (31% vs 68% for untreated animals), which are an important cause of lung damage. Overall, NV-387 treatment led to significant protection of lungs in Balb-c mice lethally infected with Influenza A H3N2 virus.
Mousepox (MPox model)
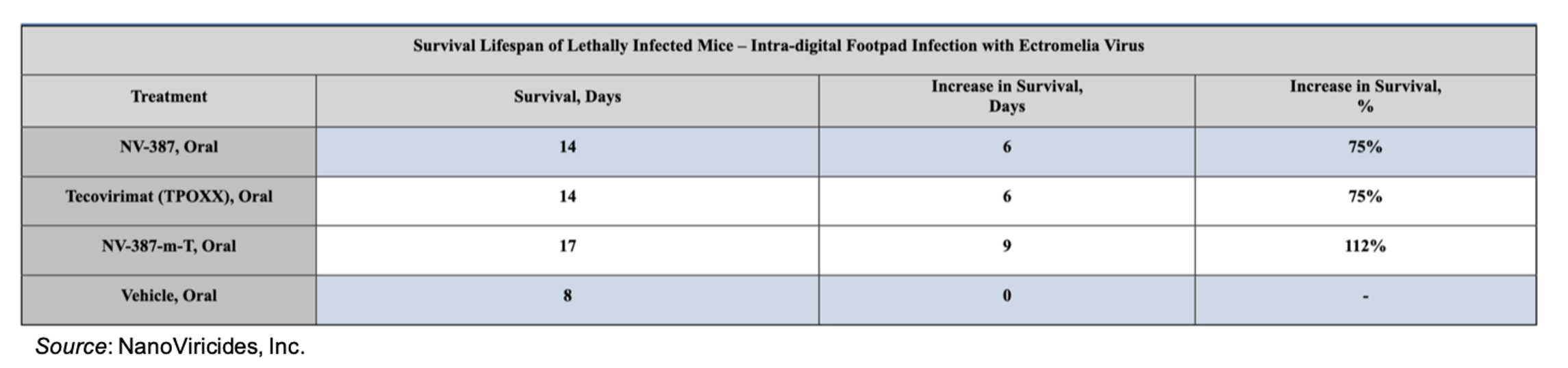
NV-387 was evaluated in a lethal model of mousepox (ectromelia) virus. The model utilizes intra-digital footpad infection, which emulates virus infection by transfer of virus via skin abrasion, a mode of MPox infection that is dominant in Western nations. NV-387 was evaluated as a monotherapy and as a combination therapy with tecovirimat (TPOXX®), which was also tested as a monotherapy. The results showed that NV-387 matched the activity of tecovirimat, which is approved for the treatment of smallpox, with both drugs increasing survival by approximately 75%. The combination therapy of NV-387 and tecovirimat led to a survival improvement of 112% compared to placebo-treated animals.
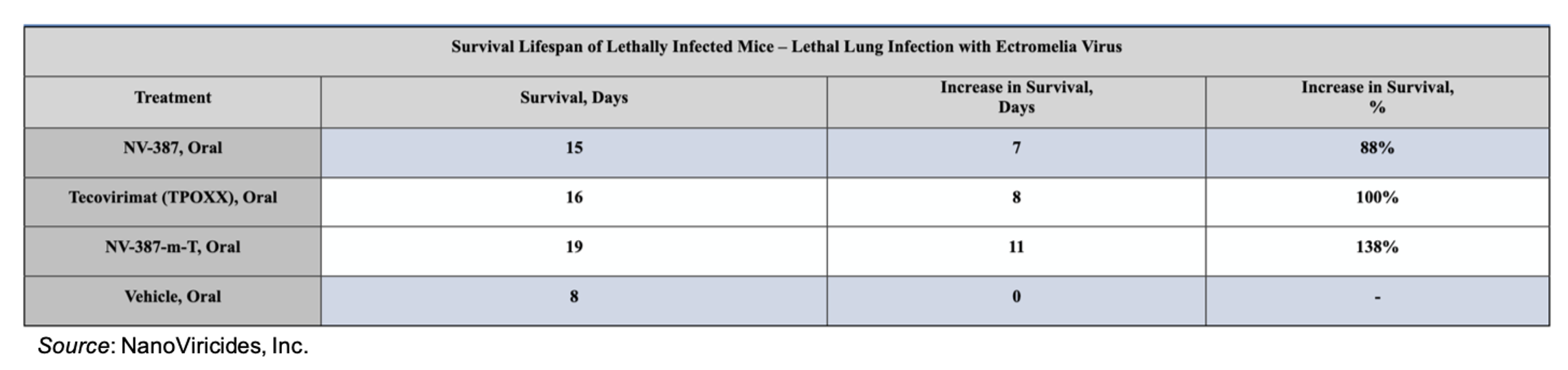
NanoViricides also evaluated NV-387 in a lethal model of mousepox lung infection. The results of that experiment are shown below. Treatment with NV-387 resulted in a similar increase in survival as tecovirimat, while the combination therapy of NV-387 and tecovirimat led to a 138% increase in survival.
Lastly, NanoViricides tested NV-387 in a lethal lung viral caused by measles virus in humanized CD150 mice. In order to cause measles infection in mice, the human CD150 receptor must be expressed on immune cells in the animals. In this model, NV-387 increased the survival of the mice to an average of 17 days, compared to 7.4 days in untreated animals.
Background on NV-387
NV-387 is based on the company’s nanoviricide® technology. It is a broad-spectrum antiviral drug that has exhibited pre-clinical activity against a wide range of viruses, including coronavirus, RSV, influenza, measles, and an orthopoxvirus model for smallpox and MPox. While seemingly disparate, each of those viruses shares a common characteristic: the utilization of heparan sulfate proteoglycan (HSPG) or sulfated proteoglycans (S-PG) as an “attachment receptor” prior to cellular infection. NV-387 is designed to mimic an essential feature of S-PG and thus act as a viral reservoir to prevent viral attachment to anchored S-PG. Given that >90% of human pathogenic viruses utilize S-PG, NV-387 has the potential to target a very broad range of viruses.
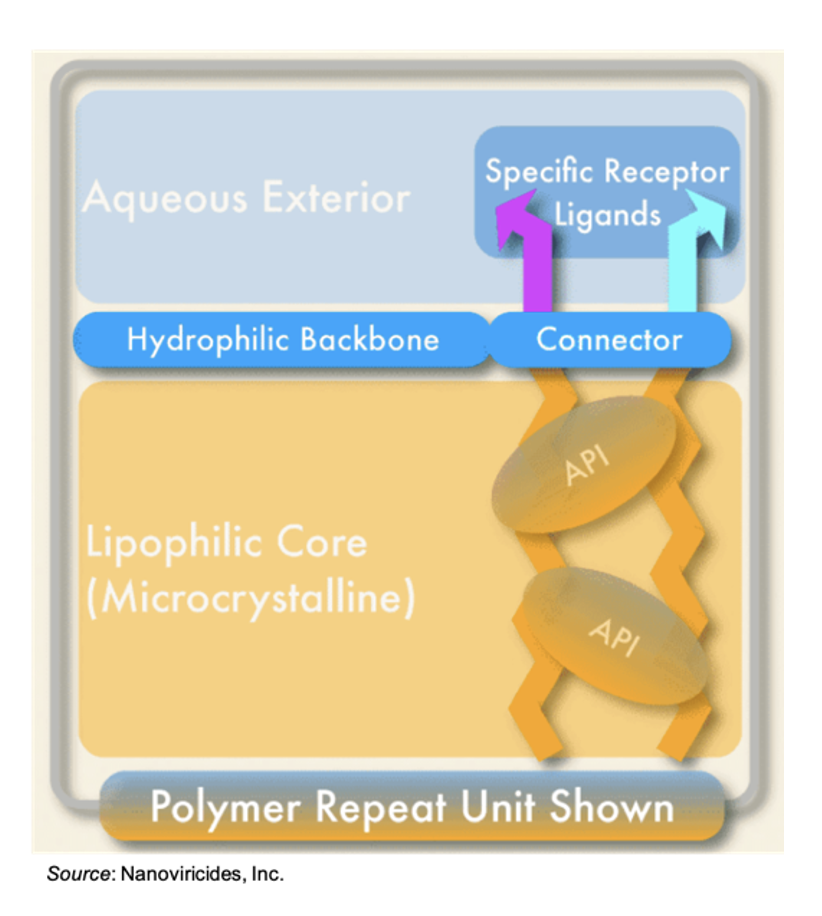
A cartoon representation of a nanoviricide is shown below. It consists of a small-molecule ligand that mimics the receptor utilized by the virus to gain cellular entry. The ligand is covalently attached to a flexible polymer backbone comprised of polyethylene glycol (PEG) and alkyl chains. The PEG forms a hydrophilic shell while also conferring non-immunogenicity. The alkyl chains make up the flexible core. Multiple chemically reactive sites allow for “packaging” of one or more active pharmaceutical ingredients (APIs) within the core of the nanoviricide. This structure then forms a flexible nanomicelle via self-assembly.
Upon encounter with a target virus, binding occurs between the ligand displayed on the nanoviricide micelle and the viral receptor protein. The micelle then fuses with the lipid-coated surface of the virus through phase-inversion and “lipid-lipid mixing”, a well-studied physicochemical effect. This is shown in the following figure. Since the binding site on the human cellular receptor for a particular virus does not change, despite mutations occurring to the receptor-binding domain and other areas of the virus, it is thought that nanoviricides will not be susceptible to viral mutations that are known to render other treatments ineffective.

The use of nanoviricides provides several key advantages over currently available antiviral therapies, including:
- Less susceptible to viral resistance: Since nanoviricides contain host-like binding domains rather than a synthetic molecule or antibody, it will likely be much more difficult for the virus to evolve resistance.
- Broad-spectrum activity: Since over 90% of human pathogenic viruses use a similar first attachment point to gain entry into human cells (Heparan SPGs), a single drug like NV-387 can potentially target many viruses.
- Host-independent action: Unlike vaccines or antibody therapies, nanoviricides do not require a functioning immune system to exert their antiviral activity.
- Complementary use: As opposed to other antiviral agents under development that only target the intracellular life cycle of viruses, nanoviricides attack the virus outside the cell and can be loaded with antiviral agents to work synergistically inside the cell as well.
- Multiple modes of administration: Nanoviricides can be administered intravenously, orally, or through inhalation. For the SARS-CoV-2 program, the company utilized oral gummies; however, for other programs, such as the treatment of severe acute respiratory infection of viral origin, the drug could be delivered intravenously to hospitalized patients.
Financial Update
On September 29, 2025, NanoViricides filed Form 10-K with financial results for the 2025 fiscal year, which ended June 30, 2025. As expected for a pre-revenue biopharmaceutical company, the company did not report any revenues for fiscal year 2025. R&D expenses were $5.5 million in fiscal year 2025, compared to $5.4 million in fiscal year 2024. The increase was primarily due to an increase in outside lab fees related to preparation for the Phase 2 CTA’s. G&A expenses were $4.0 million in fiscal year 2025, compared to $3.1 million in fiscal year 2024. The increase was primarily attributable to increased legal, accounting, and investor outreach expenditures.
NanoViricides exited fiscal year 2025 with approximately $1.6 million in cash and cash equivalents. Subsequent to the end of the fiscal year, the company raised net proceeds of approximately $1.25 million through its At-the-Market (ATM) facility. As of September 27, 2025, NanoViricides had approximately 17.4 million shares outstanding and, when factoring in warrants, a fully diluted share count of approximately 23.2 million.
Valuation
We value NanoViricides based on the potential for NV-387 as a treatment for MPox, stockpiled as a medical countermeasure in the Strategic National Stockpile as a treatment for smallpox, and as a treatment for respiratory viral infections. NanoViricides has shown that NV-387 is safe and well-tolerated in a Phase 1 clinical trial conducted in India. However, while the company has shown the drug is efficacious in a number of different viral animal models, efficacy in humans has not been established. Thus, while we are optimistic about the prospects for NV-387, investors should understand that an investment in NanoViricides is very high risk.
For treating MPox in Africa, we assume that the drug would be procured by governments and/or charitable organizations for distribution. We view the procurement and distribution of Jynneos®, a vaccine for MPox and smallpox, as a reasonable comparison to what might be expected for the sale of NV-387 in Africa to treat MPox. Bavarian Nordic, the maker of Jynneos, committed approximately 1.0 million doses to Africa via UNICEF, along with other allocations via the World Health Organization (WHO) and the E.U. We estimate those were sold for approximately $65/dose; thus, for modeling purposes, we estimate a similar sale of 1.0 million doses of NV-387 at a price of $65/dose in 2030, with additional sales totaling $200 million over the next 9 years. Using a 15% discount rate and a 25% probability leads to an NPV of $17 million.
We believe that success for NV-387 against MPox would be a positive readthrough for success against smallpox as well, thus we model for the drug to be acquired as part of the U.S. Strategic National Stockpile (SNS). In 2024, SIGA Technologies, Inc. (SIGA) reported approximately $133 million of products sales for tecovirimat (TPOXX®), with approximately $100 million being from the sale of oral and IV TPOXX to the U.S. SNS. We estimate that total sales of TPOXX to the U.S. SNS have been >$400 million since 2019. Brincidofovir (TEMBEXA®) was approved for the treatment of smallpox in 2021, and a contract signed with BARDA called for an initial procurement of 319,000 treatment courses for approximately $115 million, with options over the 10-year contract that could increase sales up to $680 million. While both tecovirimat and brincidofovir were approved for the treatment of smallpox using the FDA’s “Animal Rule”, both drugs do have shortcomings. A single point mutation in the VP-37 protein of smallpox is known to cause resistance to tecovirimat (Smith et al., 2023). Brincidofovir carries a ‘Black Box Warning’ due to an observed increase in mortality when the drug was utilized in a 24-week clinical trial for another indication, can cause diarrhea and other gastrointestinal adverse events, may cause fetal harm, and is considered a potential human carcinogen (U.S. FDA). Thus, we believe there is an unmet need for a safe and reliable smallpox therapy. We model for a 10-year contract for NV-387 to be stockpiled in the U.S. SNS for $680 million, the same as was done for brincidofovir, with the contract starting in 2030. Using a 15% discount rate and a 25% probability of approval leads to an NPV for this opportunity of $47 million. We model for international sales to equal 50% of the contract price over that same ten-year period, and using a 15% discount rate and a 10% probability leads to an NPV of $9 million.
Lastly, we model for NV-387 to be sold as an empiric therapy for the treatment of viral respiratory illnesses. We estimate peak sales of $1 billion occurring seven years after initial approval in 2033. Using a 15% discount rate and a 10% probability of approval, given that this indication is likely the farthest off and last on the priority list of the included indications, leads to an NPV for this indication of $17 million.
Combining the NPVs for each indication totals $90 million. We apply a 4x multiple and add the current cash to arrive at a total NPV of approximately $363 million. The company currently has approximately 17.4 million shares outstanding, and we estimate that it will need to sell 40 million additional shares to raise the estimated $60 million necessary for the development of NV-387 for each indication. This leads to a valuation that is rounded to the nearest whole number, of $7.00 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.