By Brad Sorensen, CFA
OTC:NRXBF
READ THE FULL NRXBF RESEARCH REPORT
NurExone (OTC:NRXBF) is developing a product known as ExoPTEN that is designed to treat patients with central nervous system injuries, which includes spinal cord damage, while also conducting preclinical tests for other conditions that ExoPTEN may be able to treat, such as glaucoma. On that latter point, the company recently announced some preclinical results that show great promise and help to further demonstrate the potential uses of ExoPTEN and how exosomes could be used as a new modality and drug delivery system that would get numerous treatment to a much more targeted area.
Before getting into these exciting results, we briefly want to touch on the 3Q financial results recently released. These results show a good cash position, a continued clean balance sheet with low liabilities and a commitment to controlling expenses—continuing the good management practices we have seen with NurExone’s throughout their history.
In a preclinical study conducted at the Goldschleger Eye Institute at Sheba Medical Center, test demonstrate that ExoPTEN’s biological activity increases with higher dosing levels. This is a significant finding as reproducibility is a key challenge, and these results confirm that ExoPTEN meets that standard. Further, functional measurements of retinal activity using scotopic threshold response electroretinography (STR-ERG) showed that both ExoPTEN doses improved visual signal strength in animals with optic nerve injury, with the high-dose group achieving response amplitudes comparable to those of uninjured eyes. This result demonstrates substantial functional recovery and, according to management, provides clear evidence of a dose-dependent therapeutic effect that aligns with ExoPTEN’s proposed biological mechanism.
The figure below depicts scotopic threshold response (STR) amplitudes measured by electroretinography (ERG) in rats subjected to optic nerve crush (ONC) and treated with exosome-based formulations. The y-axis shows STR amplitude (µV), representing retinal ganglion cell function, while the x-axis displays experimental groups. Eyes with ONC were treated with low-dose or high-dose ExoPTEN (exosomes loaded with PTEN siRNA).
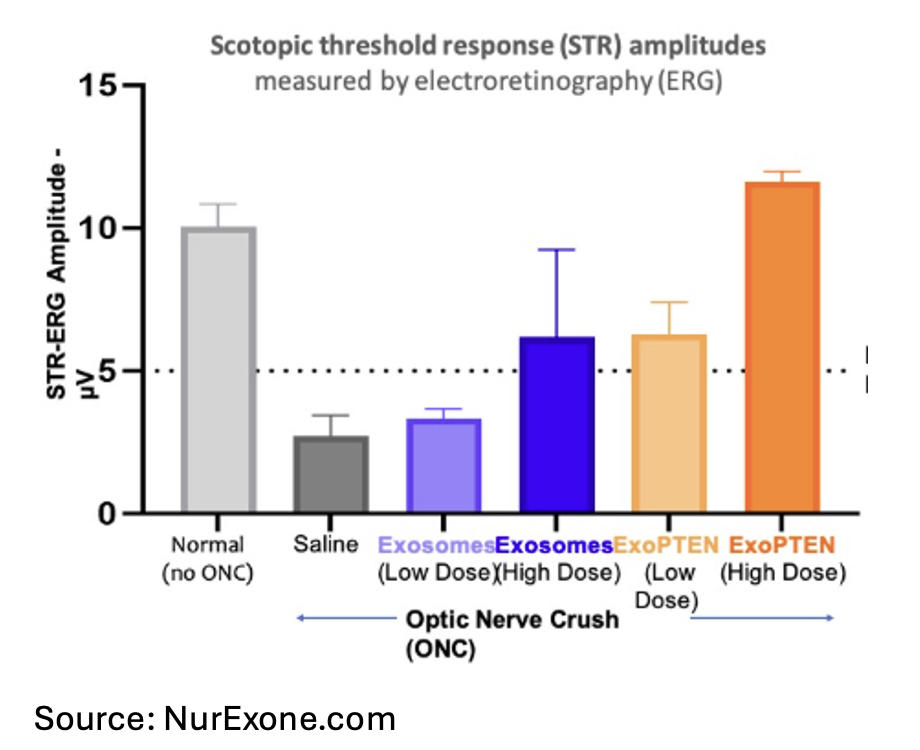
As the figure shows, low dose and high dose exosomes produced some mild improvement, but the high dose ExoPTEN treatment resulted in an extremely high recovery rate. This bodes well for its potential to target and treat conditions such as glaucoma and moves the treatment one major step closer to human trials.
Company management also recently announced that its preclinical study on ExoPTEN for the treatment of spinal cord injuries demonstrated that higher doses of the treatment led to regained motor function after a spinal cord injury. The study was conducted on small animals, which were given differing doses of ExoPTEN on the day of spinal compression surgery. The results show that 100% of animals treated with the higher dose regained walking ability in both front and hind legs, while only 1 out of 6 of the untreated animals achieved that milestone. This is an exciting result and provides further proof of the potential for ExoPTEN to be game-changing treatment.
To further the process, the company plans to initiate a Phase 1/2a clinical trial in the area of acute spinal cord injuries for ExoPTEN in 2026. Management detailed the study plans as involving adult patients with traumatic spinal cord injuries between spinal level C5 and T10. Those patients will be treated within 3-to-7-day post injury. This marks a significant step forward for the company in our view and, given the preclinical results that we have outlined, we expect the trial to yield exciting results.
The company’s ExoPTEN therapy has received the Orphan Medicinal Product Designation by the European Medicines Agency (EMA). According to the company, the EMA’s Orphan Medicinal Product Designation offers incentives, including ten years of market exclusivity upon approval, access to grants and incentives from the European Commission and member states. Additionally, the company may benefit from free or reduced-cost scientific advice and assistance with clinical trial design, which can streamline the regulatory process and reduce development costs. Lastly, some European Union countries also provide tax credits and other financial incentives to support orphan drug development.
As we’ve noted before, the company received the Orphan Drug Designation for ExoPTEN in 2023 from the FDA in the United States. This designation was created by the FDA which noted that supporting the development and evaluation of new treatments for rare diseases is a key priority for the agency. The FDA has authority to grant orphan drug designation to a drug or biological product to prevent, diagnose or treat a rare disease or condition. Orphan drug designation qualifies sponsors for incentives including:
- Tax credits for qualified clinical trials
- Exemption from user fees
- Potential seven years of market exclusivity after approval
It was earlier test results from the use of ExoPTEN that sparked our enthusiasm for the company, because the initial test results are, in our view, truly remarkable. This isn’t a potential treatment that was arrived at quickly or easily as research began at the University level and was conducted between January 2017 and May 2020, including testing the use of intranasal administration of exosomes driven from mesenchymal stem cells loaded with siRNA (a process that is described in more detail below). Testing targeted a complete spinal cord transection in rats, which is the strictest animal testing model, successfully demonstrating significant functional recovery. The company notes that the technology is successfully proven in additional preclinical studies, demonstrating that intranasal administration of ExoPTEN led to significant motor improvement, sensory recovery, and faster urinary reflex restoration. As mentioned, the research began at the University level and the Company has been granted an exclusive worldwide license from the Technion and Tel Aviv University, which includes a patent application, to develop and commercialize the technology. In addition, the Company has developed its own intellectual property and now has five families of patents.
Finally, the company announced that it was named a finalist for two highly respected international programs recognizing innovation: The Falling Walls Science Breakthroughs and the Prix Bridges Awards—further indication of the groundbreaking work NRXBF is doing.
We continue to be enthusiastic about the prospects for NurExone and suggest that US investors follow the Canadians and look into NRXBF. We urge investors with a higher risk tolerance to take a look at NRXBF and consider whether this compelling story may be of interest.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.