By John Vandermosten, CFA
NASDAQ:RADX
READ THE FULL RADX RESEARCH REPORT
RAD101 Phase IIb Interim Analysis
Radiopharm Theranostics (NASDAQ:RADX) reported the primary endpoint for its RAD101 trial in an interim analysis on December 15th. Following the release, management held a webcast which featured the company’s CEO Riccardo Canevari, Chief Medical Officer Dr. Dimitris Voliotis and the principal investigator on the RAD101 trial, Dr. Harshad Kulkarni.
Topline from the release indicated that 92% (11/12) of evaluated patients treated with RAD101 achieved concordance with Magnetic Resonance Imaging (MRI) imaging, which was the primary endpoint. RAD101 uptake was selective and significant in suspected or recurrent brain metastases.
Exhibit I – RAD101 Development
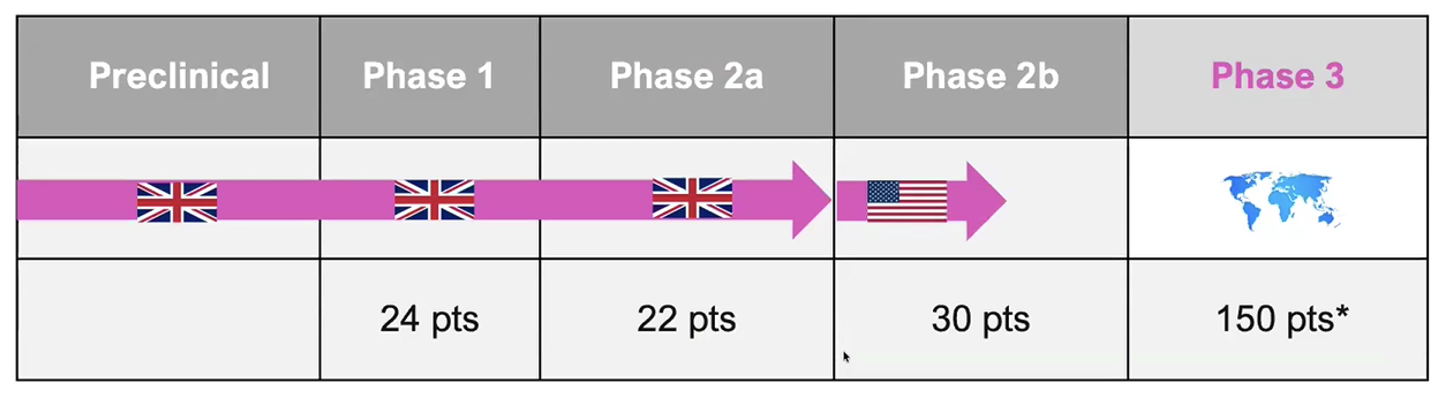
Source: Radiopharm Theranostics December 2025 Presentation
RAD101 Interim Topline Data Readout
The primary objective for the Phase IIa study evaluating RAD101 was to measure F-18 pivalate uptake in brain metastases and evaluate the impact of Stereotactic Radiosurgery (SRS) on F-18 pivalate uptake at 4 to 8 weeks. We include a summary of RAD101 in a later section of this report. The Phase IIb study for RAD101 is ongoing and recruiting subjects with an expected readout in 1H:26.
Interim results from the trial found that there was high uptake of F-18 pivalate, independent of primary tumor origin (lung, breast, melanoma and colorectal) observed in multiple brain tissues. Patients with high uptake suffered shorter overall survival (OS) (median 4 vs. 15 months with a p value of 0.0136) while MRI was uninformative. High uptake was considered to be a maximum PET Standardized Uptake Value (SUV)MAX ≥ 2.0. As an example, the following image shows agreement between the MRI scan and the PET scan. Note the bright area on the left side of each image along the horizontal centerline.
Exhibit II – Concordance Between MRI & PET
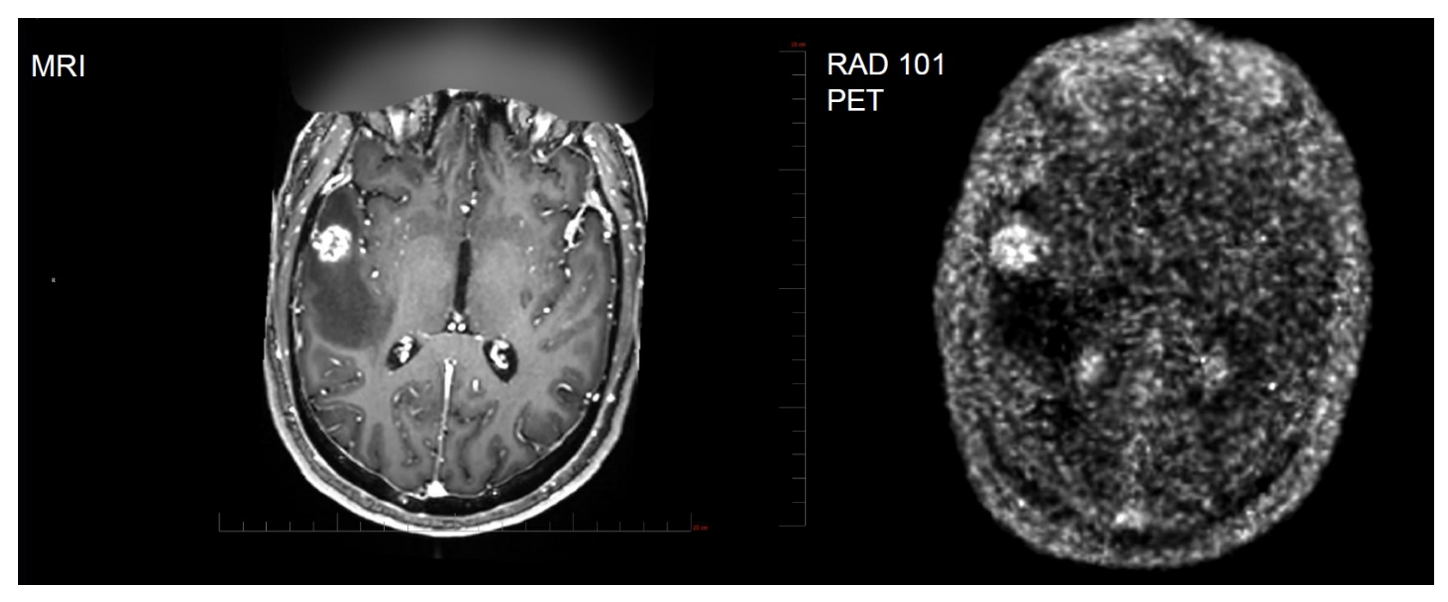
Source: Radiopharm Theranostics December 2025 Presentation
In the presentation, Dr. Kulkarni reviewed patients’ MRI and Positron Emission Tomography (PET) scan tracer uptake. While reviewing sequential images, he noted that the MRI results were inconclusive; however, the RAD101 PET scan distinctly indicated active tumor. Another subject generated MRI images that were faint and inconclusive; however, when the PET with RAD101 was examined, the tumor region was very bright and easy to distinguish.
Dr. Kulkarni further noted that the PET scans can help quantify tumor volume. Monitoring tumor size enables the oncologist to determine if the treatment is working. The presenters also differentiated between MRI and PET noting that MRI is only an anatomical picture of the brain whereas PET can show metabolic and molecular features.
The last patient image that was shared was an example of a patient with no active tumor detected using PET and RAD101. This was an example of a patient who would not need further treatment at that point in time. Some of the risks of administering SRS include pressure effects, damage to healthy tissue, nausea, headache, vomiting, brain swelling, seizures and neurological deficits. Avoiding the use of SRS when not needed can eliminate these risks and the cost of additional treatment.
In summary, Radiopharm asserts that the study is recruiting well and site engagement is high. There is a high degree of positive correlation (concordance) between MRI and RAD101 PET imaging. The study continues with longitudinal scans underway to establish the standard of truth so that final data may be generated.
Summary
Radiopharm provides an interim look at its Phase IIb RAD101 trial, generating impressive results for its primary endpoint. Results demonstrated a 92% concordance between F-18 pivalate measured by PET and MRI for the first twelve patients in its trial. While not final data, these results in 11 of 12 patients show that RAD101 in PET scans provides valuable information to oncologists that can be used to determine optimal treatment. In many cases, results from MRI are inconclusive and may delay necessary treatment or lead to further and unnecessary SRS treatment. As for next milestones, we expect to see a further readout for RAD101 in 1H:26 and the launch of a Phase III study in 2H:26. Based on the favorable data, we increase the probability of success for the RAD101 program which supports a rise in our valuation to $13.50 per ADS.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.