By John Vandermosten, CFA
NASDAQ:RADX
READ THE FULL RADX RESEARCH REPORT
Since our May initiation of Radiopharm Theranostics Limited (NASDAQ:RADX), the company has provided several progress updates. Two radionuclide supply agreements were signed, the RAD204, RAD202 and RAD101 studies have advanced, the RV01 trial reported preclinical data and received investigational new drug (IND) clearance, a research and development (R&D) tax credit was paid and Dr. Sartor was appointed to the Scientific Advisory Board (SAB). By far, the most impactful of the announcements relates to the forward movement of the clinical trials. We saw the RAD204 trial cleared to move to the next dose in its Phase I trial, the first patient dosed in the RAD202 Phase I study and the grant of the Fast Track designation for the RAD101 program for imaging brain metastases by targeting fatty acid synthase (FASN). Radiopharm also filed its quarterly cash flow and activities report for the final quarter of its 2025 fiscal year.
Quarterly Activities and Cash Report, Fiscal Year 2025
In addition to a number of business updates which we discuss below in further detail, Radiopharm Theranostics provided a financial update reviewing cash balances and allocation of cash expenditures for the period ending June 30th, 2025. The details are included in the Quarterly Activities & Cash Report.
As of June 30, 2025, the company’s cash balance was A$29.1 million, an increase from A$18.6 million a year earlier. Cash inflows rose to A$5.4 million related to the Lantheus investment while research and development cash expense was A$28.4 million. Total cash used in operating activities for FY:25 was A$36.7 million. Financing cash flows were A$44.2 million, with proceeds from issue of equity securities only partially offset by transaction costs, repayment of borrowings and other finance related expenses. Following the end of the reporting period, Radiopharm announced that it had received A$4.58 million from the Australian government related to its research and development tax incentive. We expect the annual report with audited financial statements for fiscal year 2025 next month.
Supply Agreements
ITM and Lu-177
Over the years, Radiopharm has signed several supply agreements for the radioisotopes used in its portfolio of assets. Agreements go back several years including several in 2022 for Actinium-225 and Lutetium-177. The most recent agreements were announced in the second quarter including one for non-carrier added (n.c.a.) Lutetium-177 with ITM Isotope Technologies Munich. ITM holds a US Drug Master File with the FDA for n.c.a. Lu-177 and has marketing authorization in the EU with EndolucinBeta. As described in a press release, Radiopharm will use the supply for its pipeline of Lu-177 candidates. In all, Radiopharm has four agreements for Lu-177 which further include ANSTO in Australia, SHINE in the United States and Isotopia in Israel. The agreements are expected to provide uninterrupted access to the radioisotope.
Non-carrier added Lu-177 is a highly pure form of Lu-177 which contains little to no stable lutetium resulting in higher specific activity. This allows for better binding, less competition from inactive isotopes and more effective therapy using lower doses. Radiopharm will use the Lu-177 across its clinical pipeline including RAD 204, the PD-L1 targeting nanobody, RAD 202, the HER2 targeting nanobody and RV01, a B7-H3 targeting monoclonal antibody.
Cyclotek and Tb-161
Terbium-161 is a less well-known radioisotope that emits β particles and Auger electrons. Linked with the anti-Kallikrein Related Peptidase 3 (KLK3), it is the radioligand component of RAD 402. In late June, Radiopharm signed a supply agreement with Cyclotek, a radiopharmaceutical manufacturer in Australia. The execution of the agreement is the final step required prior to requesting approval from the ethics board and launching the Phase I clinical trial in prostate cancer.
RAD 402 (Tb-161 KLK3 monoclonal antibody)
RAD 402 is in development to treat advanced prostate cancer. It links a Terbium-161 isotope to a monoclonal antibody raised to target the KLK3 gene. KLK3 encodes for this prostate-specific antigen and is highly expressed in prostate cancer cells with limited expression outside of the prostate. Another distinct feature of RAD402 is its combination with Tb-161 which emits both short-range Auger electrons and short-range β particles. These emissions allow for targeted delivery of radiation, selective cell destruction and minimal damage to surrounding tissue. KLK3 is almost exclusively expressed in the prostate, unlike PSMA which appears throughout the body. This PSMA off-target expression is responsible for some of the toxicities associated with PSMA targeting agents including Pluvicto. The goal with RAD402 targeting KLK3 is to reduce this off-target effect.
RV01 Clinical Trial
In early June, Radiopharm announced that it had compiled its preclinical data for RV01 in order to submit an investigational new drug (IND) application with the FDA. In late July, the FDA cleared the IND, allowing the trial to start. RV01 is a radiopharmaceutical candidate targeting B7-H3 (also known as CD276) using a monoclonal antibody conjugated to the β-emitting lutetium-177. B7-H3 is expressed in multiple cancers including prostate, lung, hepatocellular carcinoma, pancreatic, colorectal, head & neck and breast.
The data from the study validated earlier preclinical work that showed strong affinity to the B7-H3 target without the extensive circulation time of other monoclonal antibodies (mAbs). Compared with the half life of other mAbs which can be over one week, RV01 peaks within one or two days. The antibody is excreted by the liver more quickly which allows the radioligand to target the desired tissues, potentially without the associated toxicities that would be present with a longer duration of exposure. Biodistribution and clearance details over a week-long period for RV01 in B7-H3 positive and negative tissue and for major organs is provided in the following exhibit.
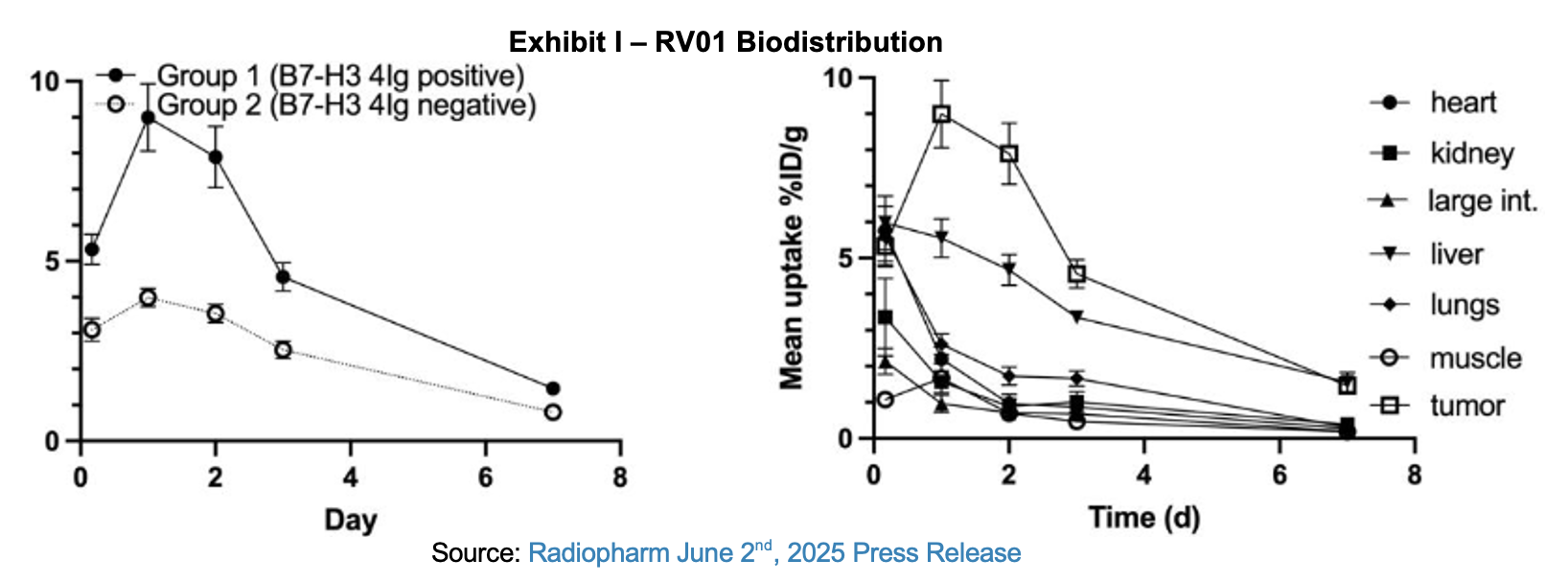
Radiopharm submitted its IND in June and announced that the FDA had cleared it on July 28th. The company plans to initiate the Phase I study for RV01 in solid tumors in 4Q:25. The company also indicated that the compound RV01 would be referenced as Betabart and further refined the description of the mAb indicating that it was designed with a strong affinity for the 4Ig isoform of B7-H3. This isoform is highly expressed in tumors but not in healthy tissues. It is further expressed on stationary tumor cells, avoiding distraction from circulating 2Ig isoforms that are shed from tumors and which may sequester the drug.
RV01 is being developed in a joint venture with MD Anderson called Radiopharm Ventures, which was created in 4Q:22. Details of the program were in a presentation included in the following press release. The effort will combine funding and executive leadership from Radiopharm and scientists from MD Anderson. Preclinical work took place in 2024 and a Phase I basket trial is expected to start in 2025.
Asset Advancement
Radiopharm has achieved several milestones for its RAD204, RAD202 and RAD101 programs. RAD204 received a positive recommendation from the data safety and monitoring committee (DSMC) allowing the associated Phase I study to continue. The first patient in the RAD202 trial was dosed in advanced HER2+ solid tumors and the FDA granted Fast Track status to RAD101 in brain metastases imaging, allowing for increased communication with the FDA and a greater opportunity for expedited treatment.
RAD204 (Lu-177 PD-L1 Nanobody)
RAD204 is a drug conjugate linking a single domain monoclonal antibody that targets programmed cell death ligand 1 (PD-L1) and the radioactive isotope Lutetium 177. The Phase I program is evaluating RAD204’s safety in treating non-small cell lung cancer (NSCLC) and other solid tumors. The recent DSMC recommendation allows the trial to move to the next highest dose. The first cohort of four patients were treated with 30mCi of RAD204 and the DSMC confirmed that there was positive safety, pharmacokinetic and biodistribution data supporting the move to the next dose level. The subsequent cohort will be treated with 60mCi of Lu-177 and should be fully enrolled in the middle part of 2025. This cohort will include patients with multiple tumor types including NSCLC, SCLC, TNBC, cutaneous melanoma, head and neck squamous cell carcinoma and endometrial cancer.
The RAD204 Phase I trial design seeks to assess safety and tolerability of Lu-177 RAD204 and to find a recommended dose for the anticipated Phase II study. The trial is listed under the designator NCT06305962 on clinicaltrials.gov. It uses a Bayesian Optimal Interval (BOIN) design for escalation and de-escalation. The Phase I was designed based on preclinical work examining biodistribution, dosimetry and pharmacokinetics with low dose Lu-177 RAD204 in organs of interest and tumors. The trial initially targeted enrollment of 23 patients to assess the safety and tolerability of RAD204 and will expand to address additional PD-L1 expressing tumors. The trial is active and recruiting at four sites in Australia, managed by the contract research organization (CRO) GenesisCare.
RAD202 (Lu-177 HER2 Nanobody)
RAD202 is a radiopharmaceutical targeting Human Epidermal Growth Factor Receptor 2 (HER2)-expressing cancers. It combines a single-domain monoclonal antibody with the radioactive isotope Lu-177 to deliver targeted radiation to cancer cells. The isotope emits β particles and γ rays, offering a half-life of 6.73 days. Lu-177 can be used for both diagnostic (γ rays) and therapeutic (β particles) purposes.
RAD202 is the subject of a Phase I basket trial evaluating safety and tolerability in HER2-positive cancers, including breast, gastric and other solid tumors. Details of the trial are available on the clinicaltrials.gov website under the designator NCT06824155. It is referred to as the HEAT trial (HER2 Antibody Therapy with Lutetium-177). Ga-68 RAD202 provides imaging for the targeted tumor while Lu-177 RAD202 represents the treatment component. The first patient was dosed this spring, as announced in a June 4th press release at the St. John of God Murdoch Hospital. The goal of the trial is to determine the recommended Phase II dose and to evaluate safety and efficacy of the candidate in HER2-expression advanced cancers.
RAD101 (F-18 Pivalate)
RAD101, also known as F-18 fluoro-pivalic acid (F-18 FPIA), is an investigational PET imaging radiotracer developed to target fatty acid metabolism in tumors. As an F-18 labeled derivative of pivalic acid, it is structurally related to fluoroacetate but incorporates a gem-dimethyl group at the C-2 position, enhancing its metabolic stability. Unlike many conventional tracers, it does not undergo defluorination in vivo, making it particularly suitable for clinical imaging applications. It can be synthesized using automated radiosynthesis platforms, facilitating broad clinical adoption.
RAD101 is specifically designed to target fatty acid synthase (FASN), a multi-enzyme protein responsible for de novo fatty acid synthesis. It is overexpressed in numerous malignancies, including gliomas, breast, oral, prostate, and ovarian cancers. FASN is especially relevant in brain metastases, where the lipid-poor microenvironment requires increased fatty acid synthesis for tumor survival and growth. A June 11th press release announced the FDA’s Fast Track designation for RAD101. Fast Track is a program offered by the FDA that allows for more frequent and earlier communication with the agency, earlier guidance on trial design, data collection and avoiding pitfalls relative to a non-Fast Track candidate. It can also allow for rolling review of a drug application and eligibility for accelerated approval and priority review. Fast Track candidates address serious or life-threatening conditions and address an unmet need. The press release continued with a target of topline results from the trial in the second half of 2025.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.