By John Vandermosten, CFA
NASDAQ:RVPH
Open Label Extension Key Opinion Leader Event
Reviva Pharmaceuticals Holdings, Inc. (NASDAQ:RVPH) held a key opinion leader (KOL) event featuring two luminaries in the schizophrenia space to discuss brilaroxazine and the RECOVER open label extension (OLE) full data set. Dr. Stephen Marder, professor of Psychiatry and Biobehavioral Sciences at UCLA and Dr. Larry Ereshefsky, retired professor of Pharmacology and Psychiatry at the University of Texas joined Reviva CEO Laxminarayan Bhat to review the data.
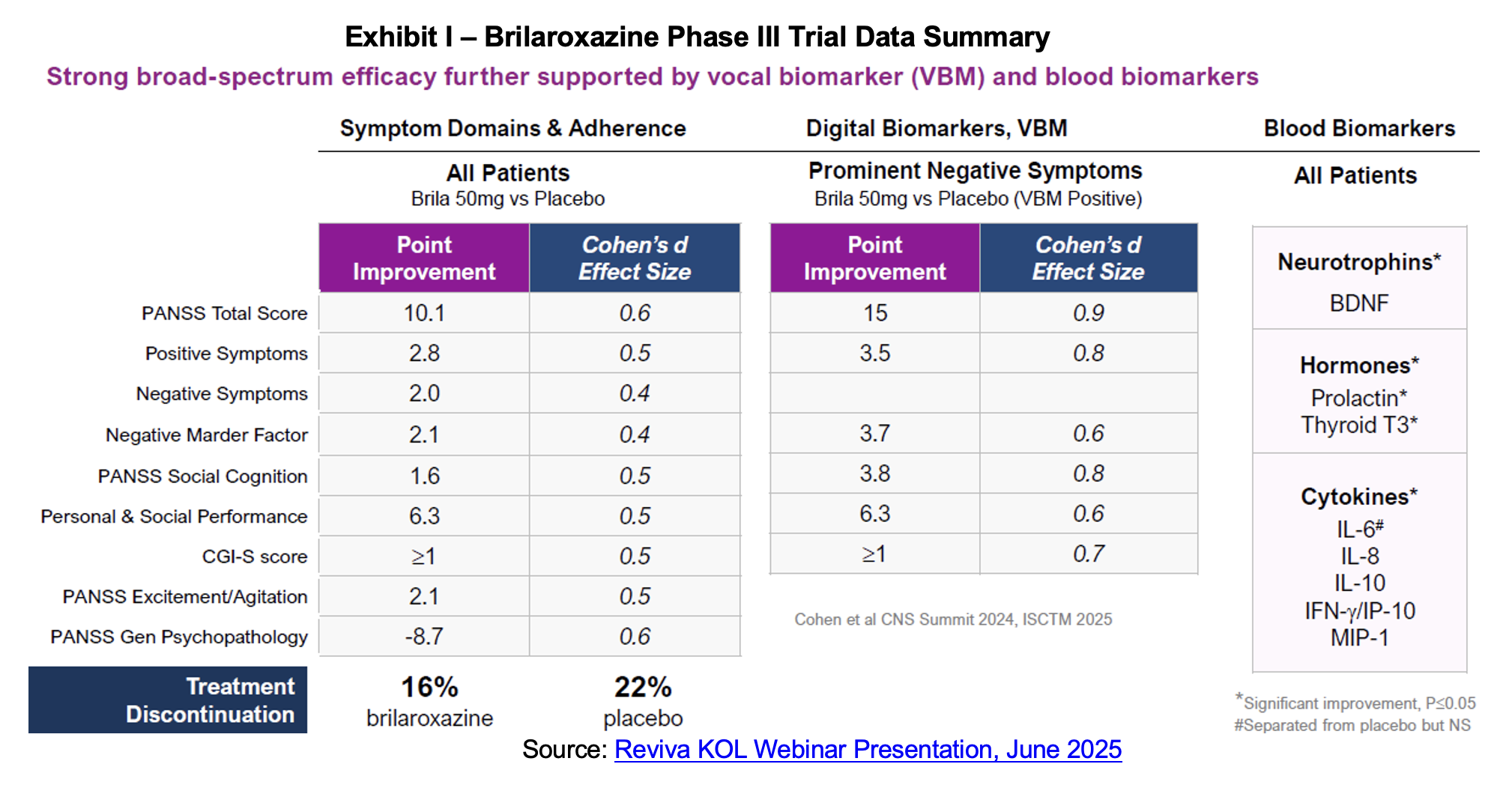
The event began with introductions of the featured KOLs and background on Reviva’s lead candidate brilaroxazine. Four trials have been run for brilaroxazine including a Phase Ia/Ib, the Phase II REFRESH trial, Phase III RECOVER trial and the OLE. The trials have evaluated 15, 30 and 50 mg doses of brilaroxazine of periods ranging from four weeks to 12 months. The drug is being developed to treat schizophrenia, a psychiatric condition that affects over 1% of the world population and an estimated 24 million people globally and 3.5 million in the United States. While there are many approved compounds to treat schizophrenia, there are also several shortcomings. Two of the most salient include addressing negative symptoms such as avolition and asociality and adherence to treatment which is impacted by unpleasant side effects.
Dr. Marder began his section of the presentation with a review of brilaroxazine’s mechanism of action, highlighting the receptor activity for the drug noting strong binding to the serotonin 5-HT2B and dopamine D2 receptors and little activity off target which is associated with negative side effects. He pointed to the large effect sizes for brilaroxazine in trials conducted to date and noted that they were sustained over a one-year period. Tolerability was another positive attribute with treatment discontinuations of only 35% over the one-year period. Based on our review of several resources,[1],[2],[3],[4] discontinuation for this class ranges anywhere from the mid-40% range to 70%. For Bristol Myers’ recently approved KarXT, discontinuation was 53% after 52 weeks of treatment.
Dr. Marder’s conclusions noted consistent, wide spectrum efficacy particularly in the negative domain, a well-run trial that generated high-quality data, a favorable efficacy to side effect ratio[5] which yields a low discontinuation rate and the potential to significantly address unmet needs.
The event continued with the presentation by Dr. Larry Ereshefsky, who has been a regular KOL contributor to brilaroxazine data analyses and who was calling in via Starlink from near the North Pole. Dr. Ereshefsky’s presentation began with a summary of the unmet needs in the space. He noted that standard-of-care antipsychotics are sub-optimal to treat chronic conditions, negative, mood and cognitive symptoms.
Unmet needs:
- Poor tolerability and prevalence of long-lasting side effects
- Recovery or remission is a rarity
- Relapse prevention is less than half by year two
- Population has high use of multiple medications and high incidences of drug-drug interactions
- Poor quality of life
- High treatment discontinuation rate
Dr. Ereshefsky reviewed the population characteristics and trial outcomes for RECOVER, noting the minimal weight gain in patients of about 1.5 kg more than placebo during the initial four weeks. At the end of 12 months, brilaroxazine subjects had gained about 1.5 kg versus baseline which was lower than the 2.4 kg gain at the end of the first four weeks. Rollover patients, who began on placebo then moved to brilaroxazine gained 1.2 kg after 13 months.
OLE results showed that the prolactin hormone, which is associated with sexual side effects, was lower for brilaroxazine subjects at the end of the trial. Pooled, 15 mg, 30 mg and 50 mg groups all showed materially lower prolactin levels compared to baseline and all were statistically significant. Elevated prolactin levels, medically termed hyperprolactinemia, represent a significant endocrinological disorder characterized by abnormally high concentrations of prolactin hormone in the blood. The clinical manifestations are diverse and primarily affect reproductive function, causing symptoms ranging from galactorrhea and menstrual irregularities in women to erectile dysfunction and reduced muscle mass in men.[6] Thyroid hormone increased in brilaroxazine subjects for all groups, with the pooled data for 446 subjects significant at a p value below 0.05. Hypothyroidism is common in schizophrenia and mood disorder patients and can further exacerbate these symptoms. Thyroid hormones are important for modulation of dopaminergic, serotonergic, glutamatergic and GABAergic receptors.
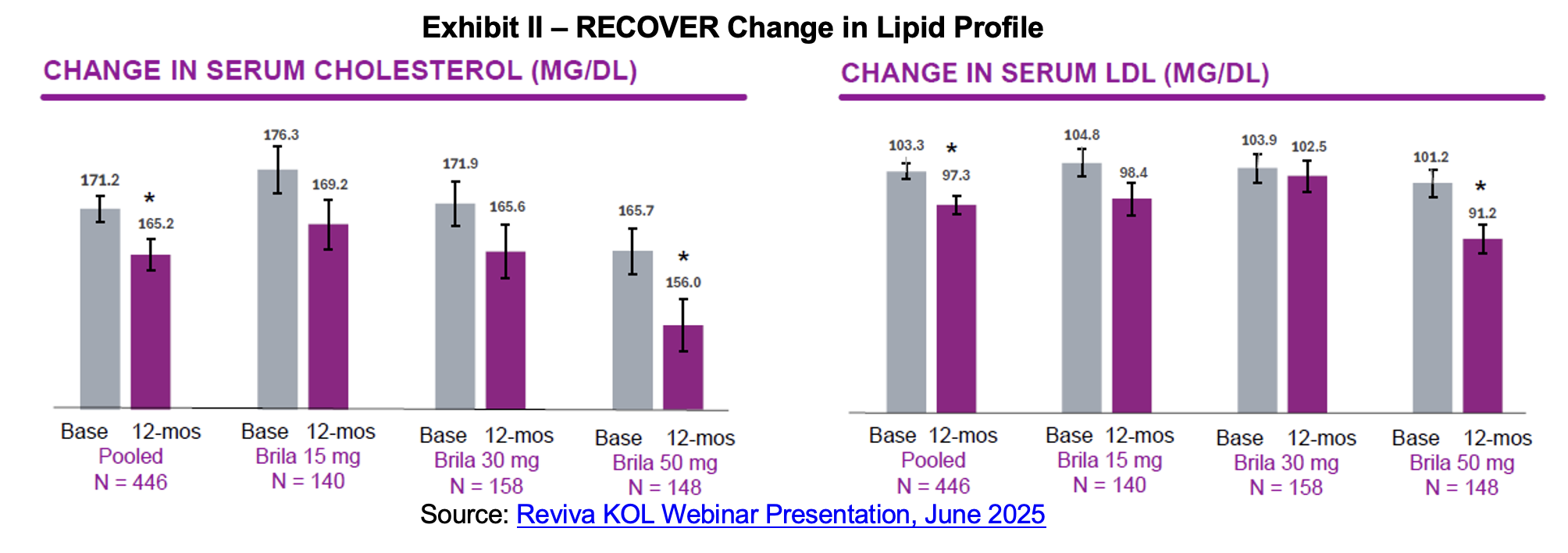
Brilaroxazine’s lipid profile is another key differentiator relative to other approved antipsychotics. For all doses, patients experienced a decline in serum cholesterol and in serum low-density lipoprotein (LDL) (bad) cholesterol. Hypercholesterolemia and dyslipidemia represent clinically significant metabolic side effects associated with atypical antipsychotic therapy, posing substantial cardiovascular risks that can dramatically impact patient morbidity and mortality.[7] RECOVER placebo patients experienced an increase in both cholesterol and LDL cholesterol over the 12-month period of 3.65 and 4.07 mg/dL respectively.
Inflammation is another area of dysregulation characteristic of schizophrenia patients. People with schizophrenia often show elevated levels of inflammatory biomarkers and research suggests that there is an association between immune system dysfunction, chronic inflammation and schizophrenia. There is a body of evidence that a meaningful proportion of schizophrenia patients are in a pro-inflammatory state where cytokine dysregulation and immune activation interplay with genetic and environmental stressors in driving the disease. Inflammatory cytokines and chemokines are thought to contribute to schizophrenia’s pathophysiology through multiple mechanisms.[8],[9]
Brain derived neurotrophic factor (BDNF) is a neurotrophin fundamental to neuronal development, synaptic plasticity, and survival. Given that schizophrenia is seen as a disorder of aberrant neurodevelopment and impaired plasticity, BDNF has attracted attention as a potential mediator in its pathophysiology. BDNF supports neurogenesis and synaptic connectivity, particularly in cortical and hippocampal circuits involved in cognition and memory. An aberration in BDNF signaling could contribute to cortical atrophy, dysconnectivity, and cognitive deficits observed in schizophrenia.[10] In contrast, higher BDNF levels are associated with clinical improvement in the disorder. Results from the OLE show that BDNF levels rose over the 12 months of the study while cytokine and chemokine levels declined.
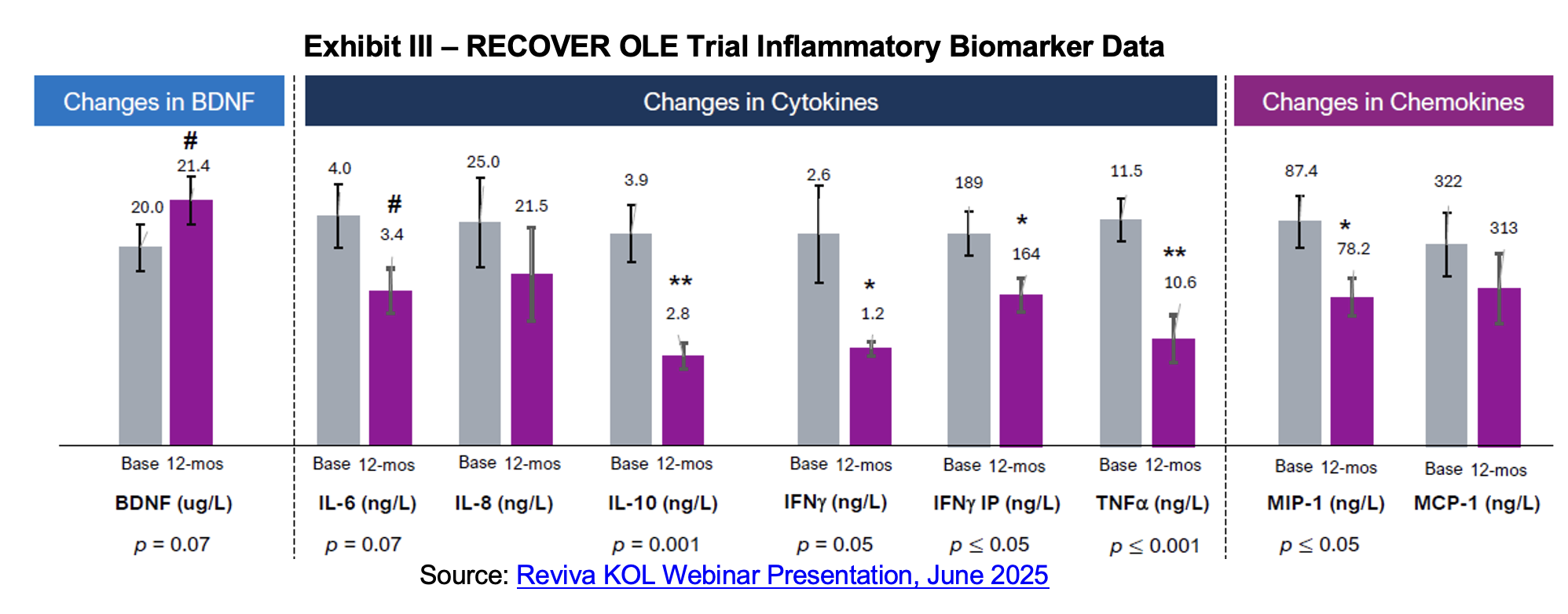
Dr. Ereshefsky summarized his assessment of the trial results noting that brilaroxazine was well tolerated at all dose strengths with minimal adverse events and low discontinuation. There were also no clinically meaningful changes in movement disorder scales used for evaluating motor side effects such as akathisia and extrapyramidal symptoms. Weight gain was minimal and moderated by the end of the twelve-month measurement period while cholesterol levels declined. No endocrine or sexual side effects were noted and elevated prolactin levels at the beginning of the trial declined by its end. Inflammation markers were also improved after a year-long course of brilaroxazine, correlated with improved PANSS scores.
At the end of the KOL event, the lines were opened for questions. These revolved around the efficacy profile of brilaroxazine compared with other antipsychotics and the biomarker results relative to other prescribed atypical antipsychotics. Other queries centered on the regulatory pathway and the need for a second Phase III study.
RECOVER Trial Background
RECOVER was a global Phase 3, randomized, double-blind, placebo-controlled, multicenter study designed to assess the safety and efficacy of brilaroxazine in 412 patients with acute schizophrenia compared to placebo. Brilaroxazine was administered at fixed doses of 15 mg or 50 mg once daily for 28 days. The primary endpoint was a decrease in the Positive and Negative Syndrome Scale (PANSS) total score compared to placebo from baseline to Day 28. Key secondary endpoints include clinical global impression (CGI) severity, positive and negative symptoms, social functioning and cognition. Topline for the trial was first announced in October 2023. The primary endpoint was met with the trial producing a 10.1-point reduction in PANSS score relative to placebo at four weeks for the 50 mg dose. Brilaroxazine also achieved statistically significant and clinically meaningful reductions in all major symptom domains and secondary endpoints at week 4 with the 50 mg dose vs. placebo. The 15 mg dose of brilaroxazine was numerically superior to placebo on the primary endpoint and most secondary endpoints, and reached statistical significance on two key secondary endpoints.
OLE Background
Following the conclusion of the RECOVER study, patients were given the opportunity to continue on brilaroxazine to gather long term safety and tolerability in an OLE study. A total of 435 patients were actively on treatment in the study across the three doses of 15 mg (139), 30 mg (155) and 50 mg (141). 156 subjects rolled over from the double-blind portion of the Phase III trial and 279 were new participants in the OLE.
The OLE was designed to take place in parallel with RECOVER and evaluate the long-term safety of brilaroxazine. To be valid, it must evaluate at least 100 subjects that were part of the RECOVER trial. The study is listed under the identifier NCT05184335 on clinicaltrials.gov in an entry that is shared with RECOVER. It evaluated flexible doses of brilaroxazine of 15, 30 or 50 mg. Data from the trial will be part of the new drug application (NDA) package that Reviva will submit to the FDA along with anticipated RECOVER-2 data.
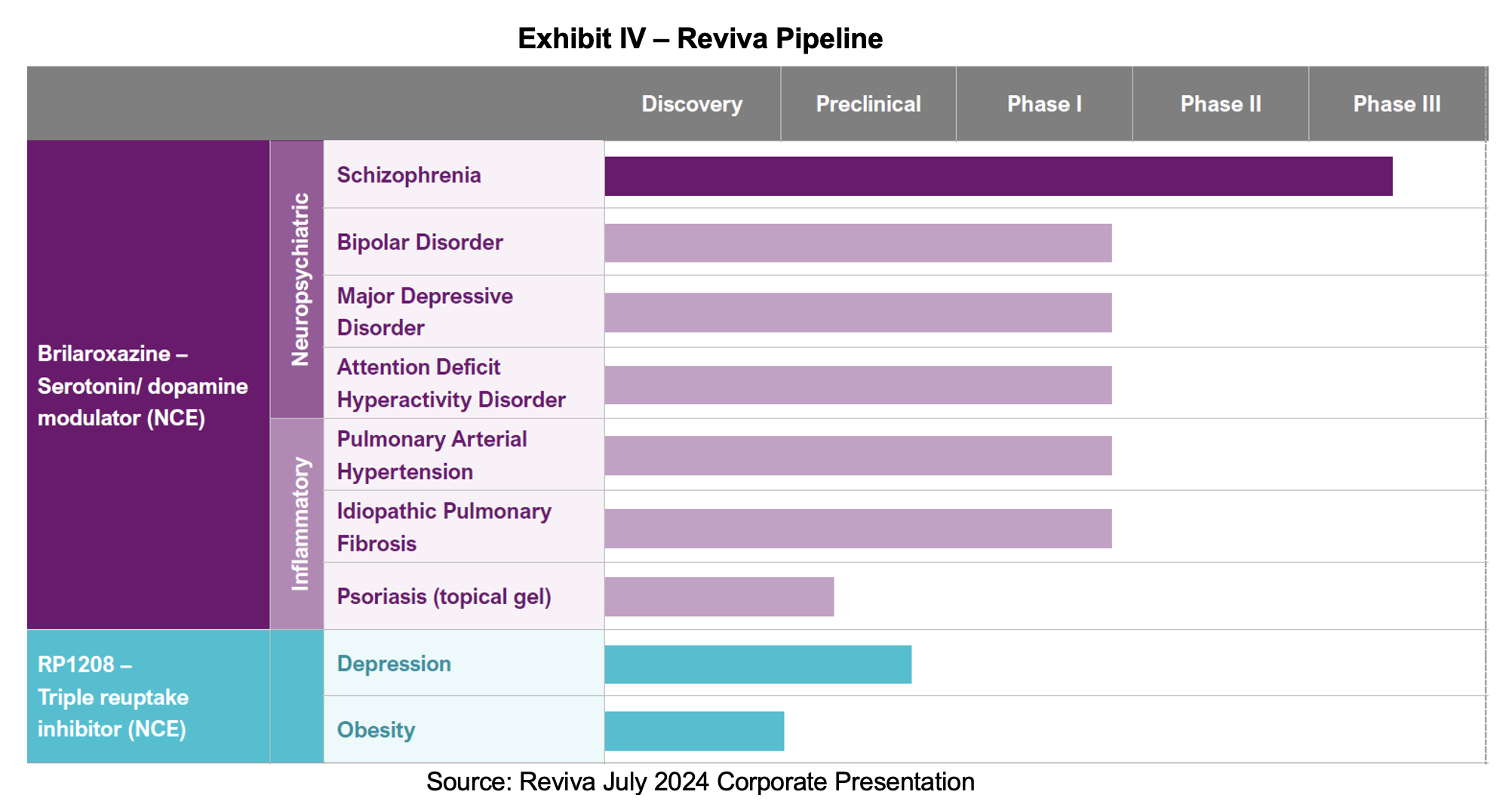
Company Pipeline
At-The-Market Facility
In a Form 8-K filed on May 30th, 2025, Reviva disclosed that it had established an at-the-market (ATM) facility with B. Riley Securities and Alliance Global Partners to offer up to $50 million in equity. Each transaction carries a 3% commission. While ATMs are helpful for covering day to day expenses, we believe the ATM will be inadequate to fund the RECOVER 2 trial and a public offering will be necessary to provide enough capital to fund the pivotal study.
Regulatory Path
Reviva is exploring the possibility of generating a new drug application (NDA) with existing data. The company has conducted a large Phase II, a Phase III and a safety study for brilaroxazine in schizophrenia. Others schizophrenia drug developers have submitted to the FDA with only one Phase III including Minerva Neurosciences with roluperidone and Intra-Cellular Therapies with Caplyta. We expect to hear additional details on this alternative in the near term following a potential FDA meeting. Skipping the second RECOVER trial would be a substantial positive for shareholders and would eliminate the overhang related to the near term capital raise necessary to run RECOVER 2.

SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
________________________
[1] Liberman, J.A., et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. New England Journal of Medicine. September 2005.
[2] Zhang, C., et al. Rates and predictors of one-year antipsychotic treatment discontinuation in first-episode schizophrenia: Results from an open-label, randomized, “real world” clinical trial. Psychiatry Research. March 2019.
[3] Bertolini, F., et al. Comparing Long-Acting Antipsychotic Discontinuation Rates Under Ordinary Clinical Circumstances: A Survival Analysis from an Observational, Pragmatic Study. Springer. March 2021.
[4] Seung-Ho, J., et al. Factors Affecting Treatment Discontinuation and Treatment Outcome in Patients with Schizophrenia in Korea: 10-Year Follow-Up Study. Psychiatry Investigation. November 2010.
[5] See our April 2024 Note that compares brilaroxazine with other leading antipsychotics: Update to Brilaroxazine Safety vs. Efficacy Comparison – Adding RECOVER Data
[6]Prolactinoma. Mayo Clinic.
[7] Carli, M. et al. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals. March 2021.
[8] Reale, M., et al. Cytokine Imbalance in Schizophrenia. From Research to Clinic: Potential Implications for Treatment. Psychiatry, March 2021.
[9] Ermakov, E., et al. Chemokine Dysregulation and Neuroinflammation in Schizophrenia: A Systematic Review. International Journal of Molecular Science. January 2023.
[10] Goren, J. Brain-derived neurotrophic factor and schizophrenia. Mental Health Clinician. November 2016.