By David Bautz, PhD
NASDAQ:SNGX
READ THE FULL SNGX RESEARCH REPORT
Financial Update
On September 29, 2025, Soligenix (NASDAQ:SNGX) announced the closing of a previously announced public offering in which the company raised gross proceeds of $7.5 million through the sale of 5,555,560 shares of common stock and warrants to purchase up to 5,555,560 shares of common stock at a combined purchase price of $1.35 per share. The warrants have an exercise price of $1.35 per share, are exercisable immediately, and expire five years from the issuance date. In addition, the company agreed that certain existing warrants to purchase an aggregate of 1,162,064 shares of common stock would be amended to have a reduced exercise price of $1.35 per share.
We estimate that this financing will extend the company’s cash runway through the end of 2026, which is past several anticipated key inflection points, as shown in the following figure.
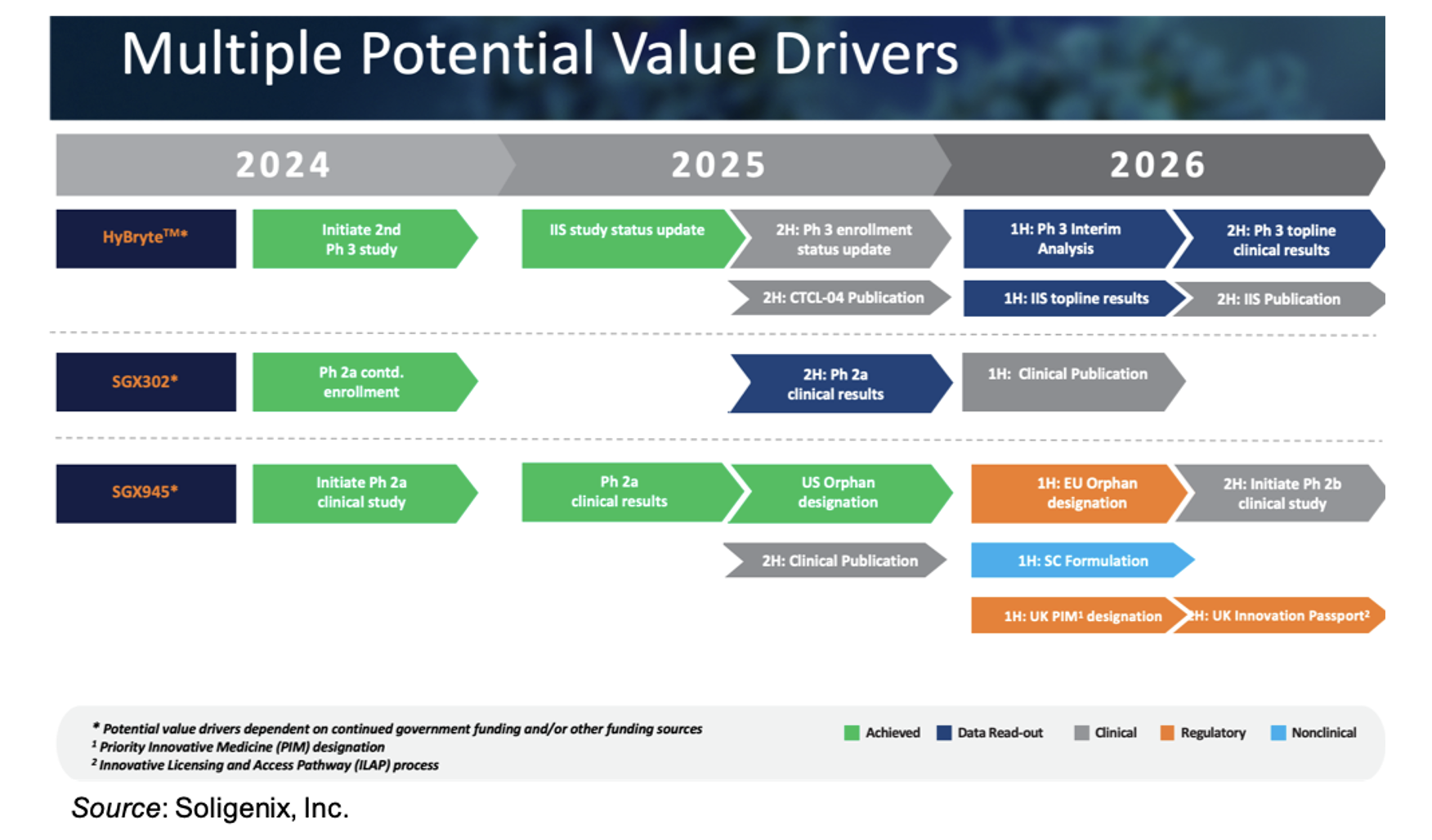
Business Update
Expands European Medical Advisory Board
On September 30, 2025, Soligenix, Inc. (SNGX) announced the expansion of its European Medical Advisory Board (MAB) with the addition of Dr. Julia Scarisbrick and Dr. Maarten Vermeer. The MAB is expected to play a key advisory role for the company in the conduct and interpretation of the upcoming Phase 3 clinical study results, which we continue to anticipate in the second half of 2026, and the associated regulatory interactions with health authorities in the E.U. and U.K. Below are brief bios of the newest members of the MAB.
Julia Scarisbrick, MBhons, ChB, FRCP, MD – United Kingdom: Dr. Scarisbrick leads the Specialist Cutaneous Lymphoma Service at University Hospital Birmingham, UK. She holds an honorary Chair as Professor for the Institute of Immunology and Immunotherapy and Dermatology Co-Lead for Dermatology Research Group, Clinical Sciences, University of Birmingham. She is the President of the ISCL, Past Chairperson of the EORTC Cutaneous Lymphoma Group, the European Director for Cutaneous Lymphoma International Consortium, the Chairperson and Trustee for the UK Photopheresis Society and Treasurer and Trustee for the UK Cutaneous Lymphoma Group (Past Chair).
Maarten H. Vermeer, MD, PhD – The Netherlands: Dr. Vermeer is the Head of the Department of Dermatology of the Leiden University Medical Center (LUMC) with over 25 years of experience in researching cutaneous lymphomas. He has more than 175 scientific publications to his name. Recently, his research activities have concentrated on clinicopathologic studies, genomic analysis of genetic and epigenetic alterations in cutaneous lymphoma tumor cells as well as international collaborative studies to develop diagnostic markers and standardize flowcytometry in cutaneous lymphomas. Dr. Vermeer served on the board of the European Society Dermatological Research, and the EORTC Cutaneous Lymphoma Working Group and chaired the board of the ISCL.
Publication Describes Extended Stability of Ebolavirus Vaccines
On September 4, 2025, Soligenix announced a publication describing the extended stability of ebolavirus vaccines using its ThermoVax® platform. The manuscript, entitled “Thermostable Bivalent & Trivalent Filovirus Vaccines from Insect Cells: Potency Demonstrated after 3 Months and 2 Years” is available online in Vaccine (Mayerlen et al., 2025). The data describes how bivalent and trivalent vaccines that were constructed from antigens against Zaire ebolavirus, Sudan ebolavirus, and Marburg Marburgvirus along with the CoVaccine HT™ antigen were formulated in a single vial and subjected to long-term storage at up to 40ºC. Following two years of storage, all vaccines were unchanged and had equivalent potency compared to when they were originally manufactured. These data confirm the advantage of a single vial subunit vaccine that can be shipped at ambient temperature and reconstituted with sterile water immediately prior to use.
Former White House Economic Advisor Appointed as Strategic Advisor
On September 23, 2025, Soligenix announced the appointment of Tomas J. Philipson, PhD as a Strategic Advisor. Dr. Philipson has expertise in U.S. economic policy, particularly health care policy, and frequently is invited to appear on major media outlets. He is currently Managing Partner of the venture capital firm MEDA Ventures. Previously, he served in a full-time position as vice chairman and acting chairman of President Trump’s White House Council of Economic Advisors from 2017 to 2020. In addition, he served as a senior economic advisor to the head of the U.S. Food and Drug Administration (FDA) and a senior economic advisor to the head of the Centers for Medicare and Medicaid Services (CMS). His vast experience and expertise will play a pivotal role for Soligenix as it advances HyBryte through its current Phase 3 clinical trial and potentially toward marketing approval and commercialization.
Conclusion
We are glad to see the company has extended its cash runway through the end of 2026, which importantly is past the expected data readout for the ongoing Phase 3 trial of HyBryte in CTCL. We anticipate data from that trial in the second half of 2026. In addition, we anticipate topline results from the Investigator-Initiated Study (IIS) of HyBryte in CTCL in the first half of 2026. Both of those expected readouts represent key inflection points that, if positive, could result in a significant revaluation of the company’s shares. We have incorporated the recent financing into our model, which has caused our valuation to decrease to $25 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.