By Steven Ralston, CFA
NYSE:VNRX
READ THE FULL VNRX RESEARCH REPORT
EXECUTIVE SUMMARY
VolitionRx’s (NYSE:VNRX): Management’s primary operational goal for 2025 is to secure multiple licensing agreements for human diagnostic applications. In September 2025, Volition began significant progress toward the goal by signing two licensing agreements: a Nu.Q NETs Research License with Werfen S.A. for Antiphospholipid Syndrome (APS) and a co-marketing agreement with Hologic Diagenode for Nu.Q Discover services.
Commercial Progress and Strategic Partnerships
Within the Nu.Q NETs pillar, the commercial strategy utilizing the CE Mark has successfully brought on 11 hospital networks across Europe in 2025. The Hologic agreement provides access to a large client base and conference marketing opportunities, with the potential to expand into an exclusive arrangement. The Werfen partnership represents the company's first human licensing deal, with the Nu.Q NETs assay successfully transferred to Werfen's AcuStar platform and an Exclusive Commercial Option Rights Agreement in place for APS.
In late-November 2025, Volition announced that Hospices Civils de Lyon (HCL) placed the company's first commercial order for Nu.Q Cancer assays. HCL is one of 14 hospital networks across five European countries scheduled to begin internal certification of Volition's Nu.Q Cancer assay prior to routine clinical use for patient disease management, particularly for Non-Small Cell Lung Cancer.

Financial Performance and Capital Management
In 3Q 2025, Volition reported total revenues of $627,277, up 32.2% year-over-year and 54.2% sequentially. Product revenues increased 32.6% year-over-year to $538,381 and 120% sequentially, primarily from Nu.Q Vet Cancer Tests and Nu.Q Discover kits sales. Service revenues increased 29.9% year-over-year but decreased 45.1% sequentially, reflecting the known lumpiness of Nu.Q Discover services revenue.
Management implemented significant cost-cutting initiatives aimed at achieving cash flow neutrality in 2025, with a stated goal of reducing annualized expenses by $10 million. Net cash used in operating activities was reduced by 33% YOY to approximately $1.2 million per month in the third quarter of 2025. Operating expenses decreased 9.7% year-over-year, primarily due to R&D expenses declining 34.2% to $958,567 following a reduction in full-time employees from 59 to 47.
Capital Raises and Share Structure
Year-to-date through September 30, 2025, shares outstanding increased 14.1% to 109,620,405 shares. Subsequent to quarter-end, Volition raised approximately $6.59 million in gross proceeds through an underwritten public offering that closed in mid-October, issuing 12,744,000 common shares and warrants.
Clinical, Technological & Educational Advancements
Volition continues advancing multiple product pillars. The Nu.Q Vet Cancer test has been successfully transferred to Fujifilm's IDS-i10 analyzer platform, an important step toward accelerating revenue growth through automation.

A Capture-Seq paper has been submitted for peer review concerning a novel liquid biopsy method achieving 100% sensitivity and specificity in identifying cancer patients; two major companies are currently evaluating the technology.
In July 2025, Volition announced the validation of point-of-care technology for NETs measurement by achieving the ability to quantitatively determine nucleosomes levels (a marker of NETosis) in whole venous blood utilizing a bedside lateral flow test. This new ability allows for diagnostic assessment of nucleosomes levels at doctors’ offices, emergency rooms and ICUs without the time delay inherent in sending a blood sample to laboratory for testing.
In October 2025, a Nu.Q Cancer poster was presented at the 47th ISOBM (International Society of Oncology and Biomarkers) Conference. The poster demonstrated that Volition's H3.1 assay has early-stage cancer detection capabilities.
Several educational webinars were conducted for Nu.Q Vet and Nu.Q Discover in the last few months.
Expected completion of clinical data for the company’s Nu.Q Feline Cancer test in 2026 will help advance the trigger for the receipt of a $5 million milestone payment based on feline product development associated with the supply agreement signed with Heska (an Antech/Mars company).
MOST RECENT NEWS
On November 25, 2025, Volition announced that Hospices Civils de Lyon (aka HCL or the University Hospital of Lyon) placed Volition’s first commercial order for Nu.Q Cancer assays. HCL is one of 14 hospital networks across five (5) European countries that are scheduled to begin internal certification process of Volition’s Nu.Q Cancer assay prior to its use in routine clinical for patient disease management, particularly for NSCL (Non-Small Cell Lung Cancer).
On September 29, 2025, VolitionRx announced the signing of a co-marketing and services agreement with Hologic Diagenode (NASDAQ:HOLX) for marketing Volition’s Nu.Q Discover services to Hologic's large client base and also at conferences & on webinars. The initial term is one (1) year; however, the agreement could expand into being an exclusive arrangement, subject to further terms being agreed upon.
Hologic Diagenode is a US$4 billion-revenue healthcare company with 44% of revenues in the diagnostics arena. Primarily focused on women’s healthcare, Hologic’s epigenomics services deal with biomarker discovery and validation with clients that conduct epigenetics research and develop new diagnostic tools in academic & public research organizations, create solutions for drug discovery and diagnostic tests at biotechnology & pharmaceutical companies and provide research services to Contract Research Organizations (CROs), all prime targets for Volition’s Nu.Q Discovery services. Nu.Q Discover provides drug developers and research scientists with assays for epigenetic profiling throughout the life cycle of drug development from disease model development through Phase III clinical studies.
On September 9, 2025, VolitionRx announced the signing of the company’s 1st human licensing deal. Specifically, the agreement is a Research License for Antiphospholipid Syndrome (APS) with Werfen S.A., which is headquartered in Barcelona and has eight Technology Centers located in Spain (1), Germany (1) and the United States (6). Under the out-licensing agreement, Werfen will have access to the components of Volition's Nu.Q H3.1 NETs assay and will investigate the assay’s clinical utility in managing APS patients. Werfen’s work will be conducted at its Immunoassay Technology Center, which is located in Lliçà d’Amunt (approximately 30 kilometers north of Barcelona). Volition’s Nu.Q NETs assay has already successfully transferred to Werfen’s AcuStar platform. Also, Volition and Werfen have entered into an Exclusive Commercial Option Rights Agreement for APS. APS is an autoimmune disorder that affects approximately four million people worldwide. The full terms of the agreement are confidential.
FINANCIAL RESULTS 3Q 2025
On November 13, 2025 after the market close, VolitionRx reported financial results for the third quarter ending September 30, 2025. Volition reported total revenues of $627,277, up 32.2% YOY and up 54.2% sequentially and above our expectations of $508,360. Product revenues increased 32.6% to $538,381 and 120% sequentially; Currently, product revenues are primarily generated from the sales of the Nu.Q Vet Cancer Tests and Nu.Q Discover kits. The company did not provide any details concerning the composition or drivers of product sales for this quarter.
Service revenues (generated by Nu.Q Discover services) increased 29.9% YOY but decreased 45.1% sequentially; Nu.Q Discover services revenue is known to be lumpy.
Management implemented a significant cost cutting initiative last year with the intent that Volition would be cash flow neutral in 2025. The stated monetary goal was to achieve a $10 million reduction in annualized expenses. In the third quarter of 2025, net cash used in operating activities was reduced by 33% YOY to roughly $1.20 million per month.
Operating expenses decreased 9.7% YOY primarily as a result of R&D expenses declining 34.2% (or by $1.19 million) to $958,567, primarily due a reduction in personnel expenses as full-time employees (FTE) decreased 20% from 59 to 47 as a result of reduced in-house clinical trial activity following completion of several studies. Sales and marketing expenses decreased by 9.0% (or $95,017); however, G&A expenses increased 36.8% (or $667,930) to approximately $2.48 million, primarily due to higher legal and professional fees (an increase of $530,308). Also contributing to the increase was a higher level of stock-based compensation (as some executives are receiving stock in compensation for reduced salaries, though this is a non-cash expense), which accounted for an increase of $207,886).
For the third quarter, VolitionRx reported a net loss of $5.38 million (or $0.05 per diluted share for stockholders) versus a net loss of approximately $5.82 million (or $0.07 per diluted share) in the comparable quarter last year.
Year-to-date through the end of the third quarter, shares outstanding have increased 14.1% to 109,620,405 shares from 96,097,485 shares on December 31, 2024. Subsequent to the quarter-end, Volition issued an additional 12,744,000 common shares associated with an underwritten public offering (see below).
RECENT CAPITAL RAISES
In mid-October 2025, VolitionRx closed an underwritten public offering of 11,550,000 shares and 5-year warrants to purchase up to 11,550,000 shares. The public offering was priced at $0.52 per set of securities. The warrants are each exercisable at $0.60 per share.
An over-allotment for 1,732,500 shares and warrants was granted (and subsequently amended) to the underwriter, Newbridge Securities, for 1,732,500 shares and 1,732,500 warrants; the over-allotment was exercised in early November in the amount of 1,194,000 shares and 1,732,500 warrants at $0.51 per share and $0.01 per warrant less an underwriting discount of 7.0%.
A total of 12,744,000 common shares were issued, and gross proceeds were approximately $6.59 million from the offering of common shares and warrants.
In mid-September 2025, Volition issued 483,870 common shares and 5-year-warrants at a combined offering price of $0.62 per set of securities to an existing stockholder in a private placement. The warrants are each exercisable at $0.682 per share. The net proceeds were $0.3 million, before deducting offering expenses of $0.02 million.
In early August 2025, Volition received net proceeds of $1.21 million from a registered direct offering. The offering consisted of 1,734,375 common shares and 1,734,375 5-year warrants to the public and 156,250 shares and warrants to certain of directors and executive officers. The public offering was priced at $0.64 per set of securities. The warrants are each exercisable at $0.768 per share.
In May 2025, Volition issued a $7,500,000 Senior Secured Convertible Promissory Note to Lind Global Asset Management XII LLC. Net proceeds were $5,802,799.
NU.Q MILESTONES ACHIEVED THUS FAR IN 2025
Nu.Q Vet
- The Fujifilm Vet Systems made substantial progress during the 3Q 2025 in validating and verifying the Nu.Q Vet cancer test on its automated IDS-i10 analyzer platform. Automation of processing Nu.Q assays at centralized labs is crucial to accelerating Volition’s revenue growth rate.
- During 2Q 2025, the first study to report the detection of nucleosomes in cats was completed; this pre-analytics work paves a path for the potential of cancer screening and monitoring in cats. The development of the Nu.Q Vet Feline Cancer Test triggers a $5 million milestone payment.
Nu.Q Discover:
- During 2Q 2025, for the first time, Nu.Q Discover biomarkers will be utilized in a human clinical study, namely in a Phase 1/2b clinical trial by an unnamed leading pharmaceutical company.
Human Diagnostics Agreements:
- In September 2025, Volition secured two significant licensing agreements
- signed company’s 1st human research Nu.Q NETs licensing deal with Werfen S.A. for Antiphospholipid Syndrome (APS)
- the Nu.Q NETs assay has been successfully transferred to Werfen’s ACL AcuStar platform
- signed co-marketing and services agreement with Hologic Diagenode for marketing Volition’s Nu.Q Discover services to Hologic's large client base
- Hologic made its first Volition-related sale during the early part of 4Q 2025
- The company continues to be in discussions with approximately 10 leading companies. The discussion stage levels range from due diligence to contract finalization.
Lung Cancer:
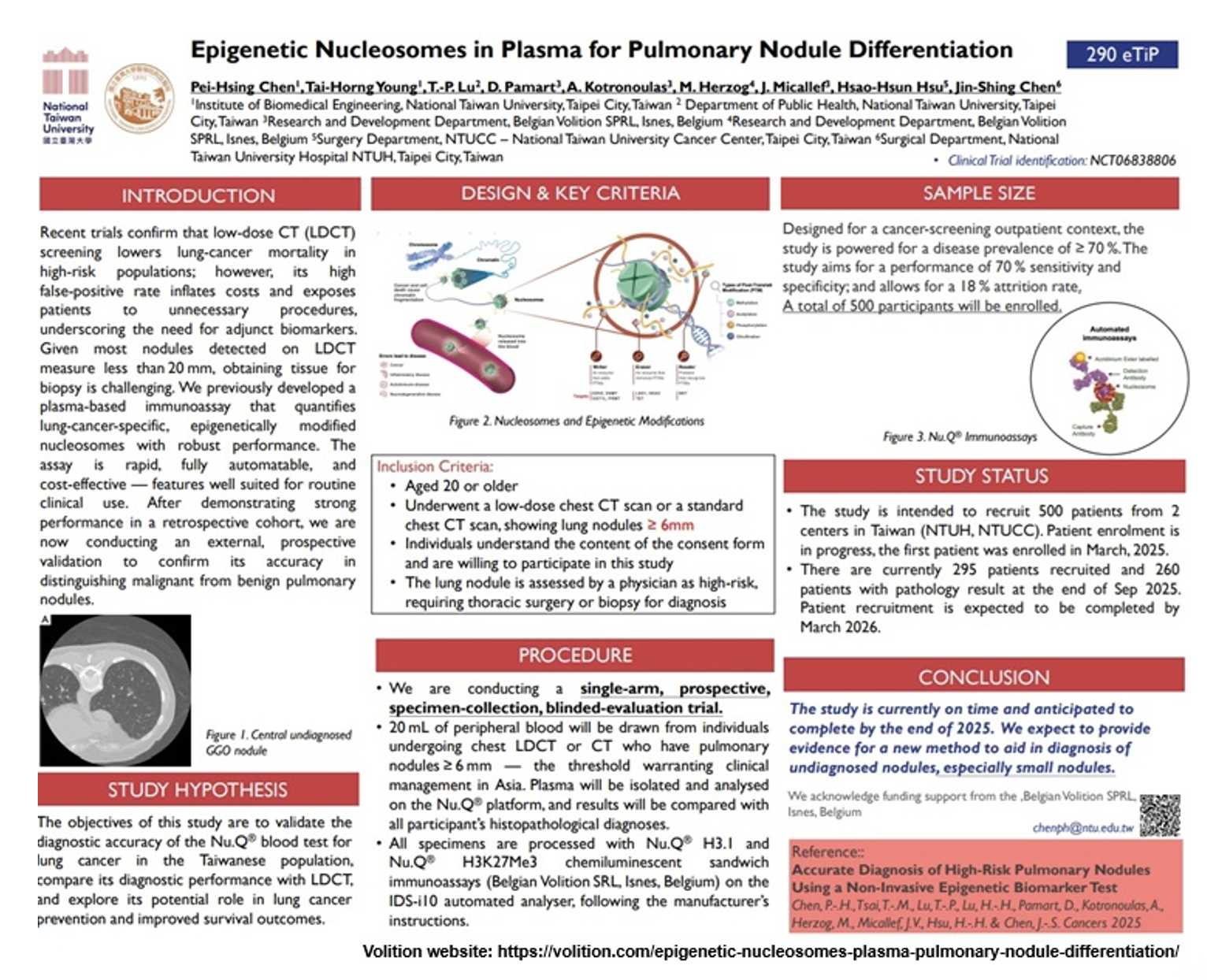
- During 3Q 2025, an IDS-i10 analyzer was installed at National Taiwan University Hospital in Taipei in order to help conduct the lung cancer screening validation study.
- During late-2025, the Nu.Q NETs Cancer assay are scheduled to begin an evaluation process at 14 hospital networks across five (5) European countries. Currently, the Nu.Q Cancer assays are in the internal certification process at the hospitals prior to use in the routine clinical use for lung cancer patient management.
- The Hospices Civils de Lyon placed Volition’s first commercial order for Nu.Q Cancer assays in late-November 2025 in order to complete its certification process.
- The Hospices Civils de Lyon is in the process of completing the long-term follow-up of patients in the ONCOPRO study (NCT03787056), a large 506 -patient, prospective, case-control study that is assessing the diagnostic and prognostic values of circulating nucleosomes in lung cancer.
Capture-Seq:
- Submitted a scientific Capture-Seq paper for peer review (see the RECENT DEVELOPMENTS: PAPERS, POSTERS Section below) that describes a groundbreaking new liquid biopsy method that removes 99.5% of the background DNA, thereby isolating and concentrating 48% of the targeted cancer-derived DNA sequences that are bound to a transcription factor (specifically CTCF) that results in an 180-fold concentration (18,000% enrichment) of the targeted circulating tumor DNA (ctDNA). The results identified patients with cancer with 100% sensitivity and specificity. The next step is to establish a proof of concept for cancer detection by using Capture-Seq.
European NET Applications based on CE Mark:
- During 1Q 2025, the first revenue recorded from the sales of a regulated, clinically approved product, specifically CE Marked Nu.Q NETs product from hospital networks in Europe.
- During 1Q 2025, Volition completed the first commercial sale of High Throughput Synthetic Sepsis Model that enables real-time measurement of NETs activation and inhibition in whole blood, which supports the development of new NETs-related disease therapeutics.
- Revvity is now selling Volition’s Nu.Q assays in Europe for use in analyzing 21 different disease applications.

EXPECTED NU.Q MILESTONES IN 2025 AND BEYOND
Nu.Q Pillars
- In the coming quarters, management also anticipates additional peer-reviewed papers across all the company’s pillars (Nu.Q Cancer, Nu.Q NETs, Nu.Q Discover, Nu.Q Vet, Capture-Seq), including papers from French collaborators. A Capture-Seq-related paper is highly anticipated.
Nu.Q Vet
- Operationally, management is focusing on ensuring that the Nu.Q Vet Cancer test is added to the annual pet wellness panels and coaxing large veterinary customers to implement centralized lab automation so that increases in sales volumes can be handled.
- The development of the Nu.Q Vet Feline Cancer Test triggers a $5 million milestone payment from Antech, which could occur in late 2025 or the first half of 2026.
Human Diagnostics Agreements:
- Having made its first sale during the early part of 4Q 2025, Hologic will contribute to Volition’s reported fourth quarter revenue.
- There is the potential that Hologic could enter into an exclusive licensing agreement for Nu.Q Discover services the initial one-year co-marketing agreement.
- Management anticipates securing additional licensing agreements for Nu.Q NETs in diagnostic applications in human cancer and sepsis.
Lung Cancer (Taiwan and France):
- National Taiwan University Hospital team is progressing with a prospective final validation lung cancer screening study with 500 patients with low dose chest CT scans (LDCT) that display lung nodules ≥ 6mm. The study is designed to recruit 500 patients. As of the end of September 2025, 295 patients have been recruited; patient recruitment is expected to be completed by March 2026. The study is anticipated to have been completed by the end of 2026 with a performance of at least 70% sensitivity and specificity. In addition, the team expects to provide evidence for a new method to aid in diagnosis of undiagnosed nodules, particularly small nodules. (see the RECENT DEVELOPMENTS: PAPERS, POSTERS Section below).
- Management expects the first clinical use of the oncology platform in France in late 2025 or early 2026. The Hospices Civils de Lyon appears to be in the lead, having placed an order for Nu.Q Cancer assays in late-November 2025 for its certification process for the diagnosis of NSCLC.
- New lung cancer data will be presented at the North American Conference on Lung Cancer (NACLC 2025) to be held in Chicago from December 5-7, 2025. The event is jointly organized by IASLC (International Association for the Study of Lung Cancer) and ASCO (American Society of Clinical Oncology).
Capture-Seq:
- Two major companies are currently evaluating Volition’s Capture-Seq technologies; the first evaluation results are expected in late 2025 or early 2026.
- The development of additional Capture-Seq applications (using transcription factors other than CTCF) for early cancer detection and patient management will be pursued in 2026.
European NET Applications based on CE Mark:
- Clinical utility studies are expected to begin and/or to have been completed across 14 hospital networks in five (5) European countries for various NETosis applications by mid-2026.
RECENT DEVELOPMENTS: PAPERS, POSTERS, WEBINARS ETC.
Nu.Q Foundational Breakthrough (Initial Target: Sepsis)
On July 8, 2025, Volition announced that it achieved the ability to quantitatively determine nucleosomes levels (a marker of NETosis) in whole venous blood utilizing a bedside lateral flow test. The blinded study of 25 hospital patients at the point-of-care demonstrated that strongly correlated with results from Nu.Q nucleosome assays processed at a central laboratory. This new ability allows for diagnostic assessment of nucleosomes levels at doctors’ offices, emergency rooms and ICUs without the time delay inherent in sending a blood sample to laboratory for testing. This project is granted by the Wallon Region.
https://volition.com/point-of-care-quantification-of-h3-1-nucleosomes-in-fresh-whole-blood-a-novel-tool-for-nets-measurement-in-hospital-setting/
Nu.Q (poster): On October 6, 2025, the lateral flow results from the paper above was shared as a poster. This breakthrough technique allows for sepsis patients in a hospital setting to have their nucleosome levels monitored almost in real time so that appropriate therapeutic actions can be made.
https://volition.com/wp-content/uploads/2025/10/2025-Sepsis-Update-Poster-Pamart-et-al.pdf
Nu.Q Vet – Veterinarian Support
In mid-July, Volition provided a webinar on its website titled “Early Detection with Canine Cancer Screening”, in which Dr. Sue Ettinger (aka Dr Sue Cancer Vet) presented clinical data from studies of the Nu.Q Vet Cancer test and explained how to process samples, interpret results and communicate results to pet owners. Case studies were provided. The webinar is available in 22 languages.
https://volition.com/early-detection-with-canine-cancer-screening/

Also in mid-July, Volition sponsored another webinar titled “Utilizing the Nu.Q Vet Cancer Test in Practice”, in which Dr. Tom Butera, DVM & CEO of Volition Veterinary, presented how to process Nu.Q Vet Cancer test samples, interpret results and how to integrate the use of the test in a veterinarian’s clinical practice. Case studies were provided. This educational webinar is designed to help veterinary practitioners understand how to effectively incorporate the Nu.Q Vet Cancer test into their clinical practices for canine cancer screening. The webinar is also available in 22 languages.
https://thewebinarvet.com/videos/utilizing-the-nuq-vet-cancer-test-in-practice
In early-October 2025, Volition supplied a support document titled “High Nu.Q Vet Cancer Test Result Guide” for veterinarians to aid in the evaluation of results of the Nu.Q Vet Cancer test, along with the suggested next steps in the diagnosis process.
https://volition.com/wp-content/uploads/2025/10/Guide.pdf
In mid-November, Volition provided a collection of Nu.Q Vet Cancer test promotional/informational materials for veterinarians to use for the purpose of educating pet parents in the UK and Europe, including posters, a trifold brochure, an informational postcard and social media assets.
https://volition.com/veterinary-resources-in-clinic-uk-eu/

Nu.Q Discover
Nu.Q Discover (clinical paper): On August 6, 2025, a paper titled “Quantification of H3.1-nucleosomes using a chemiluminescent immunoassay: A reliable method for neutrophil extracellular trap detection” by M. Wargnies et al was published in PLOS One. The study developed and analytically validated that a chemiluminescent immunoassay can measure the level of circulating H3.1-nucleosomes in plasma and further concluded that the detection of H3.1-nucleosomes by any immunoassay is a potential breakthrough method for “objective, robust, reproductible and quantitative” detection of NETs.
https://volition.com/quantification-h3-1-nucleosomes-using-chemiluminescent-immunoassay-a-reliable-method-neutrophil-extracellular-trap-detection/
Nu.Q Discover (Webinar): On October 8, 2025, Volition made available a 55-minute on-demand on GenomeWeb titled "Beyond the Genome: Measuring Epigenetic Modifications Across Matrices for Biomarker and Drug Discovery." The webinar showcases Nu.Q Discover's capabilities in measuring epigenetic modifications for biomarker discovery and drug development applications.
Nu.Q Discover (presentation - ELBS):): On November 5, 2025, at the ELBS (European Liquid Biopsy Society) General Assembly 2025, Volition’s colleagues provided an introduction to Nu.Q Discover services , explaining the scope of utilization over the phases of drug development from discovery to late clinical trials. Also, there was a brief update on the ongoing Lung Cancer study being conducted in Taiwan.
Nu.Q Cancer
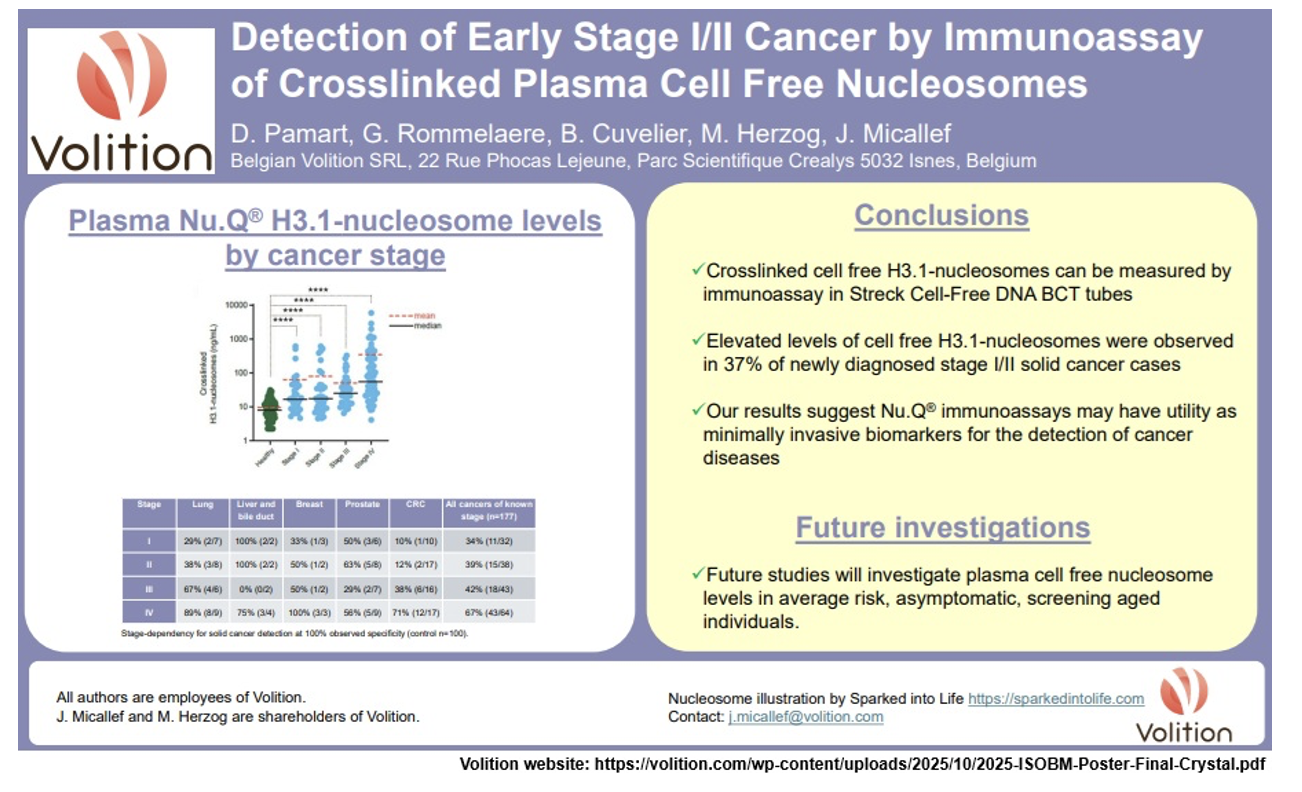
Nu.Q Cancer (poster - ISOBM conference): On October 13, 2025, a poster titled "Detection of Early Stage I/II Cancer by Immunoassay of Crosslinked Plasma Cell Free Nucleosomes" by Dorian Pamart et al was presented at the 47th ISOBM (International Society of Oncology and Biomarkers) Conference held October 13-15, 2025 in Murnau, Germany. The poster concludes that 37% of early-stage cancers were detected by elevated levels in Volition's H3.1 assay, which could aid in earlier intervention.
https://volition.com/early-stage-cancer-detection-immunoassay-cell-free-nucleosomes/
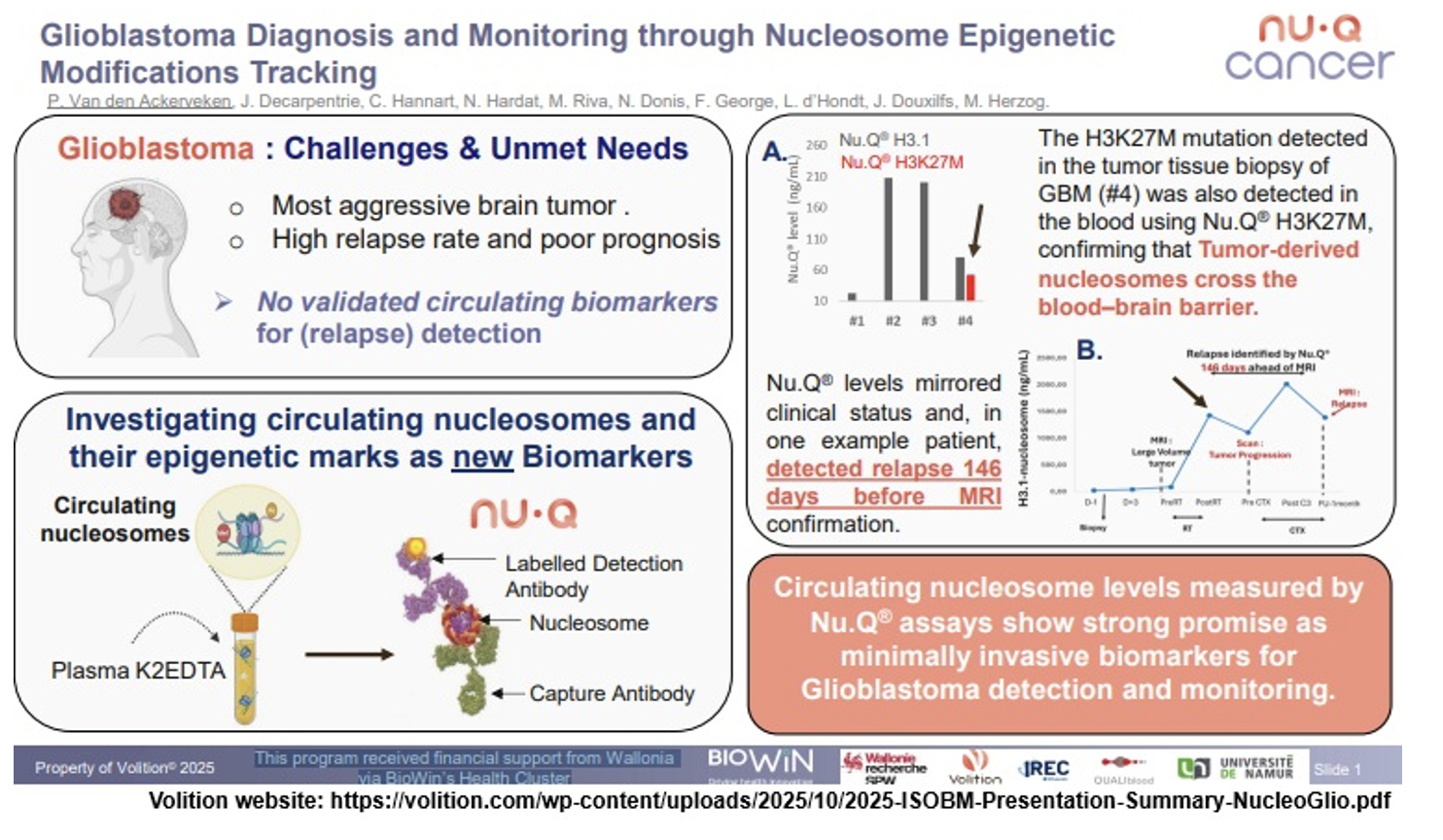
Nu.Q Cancer (presentation - ISOBM 2025): On October 15, 2025, presentation titled "Glioblastoma Diagnosis and Monitoring through Nucleosome Epigenetic Modifications Tracking" by P. Van den Ackerveken et al was given at the ISOBM 2025 Conference. A study concerning glioblastoma, an aggressive form of brain cancer, indicates that nucleosome levels detected by the Nu.Q H3.1 assay mirrored the clinical status of the patients, and that the Nu.Q H3K27M assay detected a relapse 146 days before it was confirmed by an MRI. The research program received financial support from the Wallon Region via BioWin’s Health Cluster.
https://volition.com/glioblastoma-diagnosis-monitoring-nucleosome-epigenetic-tracking/
Lung Cancer:
Nu.Q Cancer (poster – ESMO 2025): At the European Society of Medical Oncology Congress held October 17-21, 2025 in Berlin, a poster titled “Epigenetic Nucleosomes in Plasma for Pulmonary Nodule Differentiation” by Pei-Hsing Chen et al (National Taiwan University Hospital team) was presented. In this interim analysis of a prospective final validation lung cancer screening study, after demonstrating strong performance in a retrospective cohort, we are now conducting an external, prospective validation to confirm its accuracy in distinguishing malignant from benign pulmonary nodules.
https://volition.com/epigenetic-nucleosomes-plasma-pulmonary-nodule-differentiation/
Capture-Seq:
Nu.Q Capture-Seq (paper): A scientific Capture-Seq paper has been submitted for peer review. The paper describes a novel liquid biopsy method that removes 99.5% of the background DNA, thereby isolating and concentrating 48% of the targeted cancer-derived DNA sequences that are bound to a transcription factor (specifically CTCF) that results in an 180-fold concentration (18,000% enrichment) of the targeted circulating tumor DNA (ctDNA). The results identified patients with cancer with 100% sensitivity and specificity. The next step is to establish a proof of concept for cancer detection by using Capture-Seq.
VALUATION
Utilizing a financial model based on DCF methodology, which forecasts out to 2031, and uses a 10% discount rate (based on CAPM), a 2% terminal growth rate and a terminal P/S multiple of 0.48, the indicated value of VNRX is $2.50 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.