By David Bautz, PhD
NASDAQ:ARWR
READ THE FULL ARWR RESEARCH REPORT
Business Update
Encouraging Early Data for Obesity Programs
On January 6, 2026, Arrowhead Pharmaceuticals, Inc. (NASDAQ:ARWR) announced preliminary results for the company’s investigational treatments for obesity, ARO-INHBE and ARO-ALK7. The company also held a webinar to discuss the results. The slides and the webinar can be accessed here. The data showed that ARO-INHBE, both as a monotherapy and in combination with tirzepatide led to reductions in visceral fat, total fat, and liver fat. These were early data; thus, the number of patients treated so far is small, however, the early signals look promising for each of the assets.
With the success of the different GLP-1 medications already on the market, some may question if there is a need for additional obesity therapeutics. Dr. Carel le Roux presented on the unmet need in obesity treatment. Much like other chronic diseases have targets that doctors encourage their patients to achieve (e.g., <6.5% for HbA1c, <130/80 for blood pressure, etc.), Dr. le Roux and colleagues proposed obesity targets that were back-tested and found that a waist-to-height ratio (WHtR) of <0.53 and/or a BMI <27 kg/m3 after weight loss were found to decrease the risk of T2D, hip/knee osteoarthritis, and atherosclerotic cardiovascular disease (ASCVD). Interestingly, while both semaglutide and tirzepatide cause significant weight loss, based on results from the SURMOUNT-5 study (Aronne et al., 2025), only 14.2% (semaglutide) and 23.1% (tirzepatide) of patients from that study hit the aforementioned targets. This is important, as >50% of patients that achieved WHtR of <0.53 and/or BMI <27 kg/m3 had normalization of cardiometabolic parameters as well.
Additionally, even when only examining weight loss, diabetic patients consistently underperform non-diabetic patients, thus suggesting that those patients are achieving the WHtR and BMI targets at an even lower rate. This means that those individuals are going to need additional options to help them achieve the targets. While there are a very large number of MOAs being tested in the clinic, targeting the Activin E-ALK7 pathway represents a novel means to target adipose tissue.
ARO-INHBE targets the INHBE gene that encodes activin E, which is a ligand for ALK7. ARO-ALK7 is designed to reduce the ALK7 receptor, which binds activin E, and is a TGF-β superfamily member that is expressed in adipocytes.
Support for targeting INHBE derives from two papers that came out in 2022 that described loss of function mutations in INHBE that were associated with favorable fat distribution. Deaton et al. reported a genome-wide association study (GWAS) from 362,679 individuals that showed a predicted loss of function variant in INHBE associated with a lower waist-to-hip ratio adjusted for BMI (WHRadjBMI), which they used as a surrogate for abdominal fat that is causally linked to type 2 diabetes and coronary heart disease (Deaton et al., 2022). Akbari et al. performed a GWAS in 618,375 individuals and identified an association with favorable fat distribution, favorable metabolic profile, and protection from type 2 diabetes for heterozygous protein-truncating mutations in INHBE (Akbari et al., 2022). Arrowhead has conducted studies of Inhbe silencing in mouse obesity models, with results showing reduced weight gain compared to controls. Importantly, the difference in weight gain was primarily due to changes in fat mass with no difference in lean mass.
Similar to the data supporting targeting INHBE, a GWAS study in 2019 showed that four variants in the ACVR1C gene (which encodes ALK7) were associated with reduced percent abdominal fat in DEXA imaging, a lower WHRadjBMI, and a decreased risk of developing type 2 diabetes (Emdin et al., 2019).
ARO-INHBE
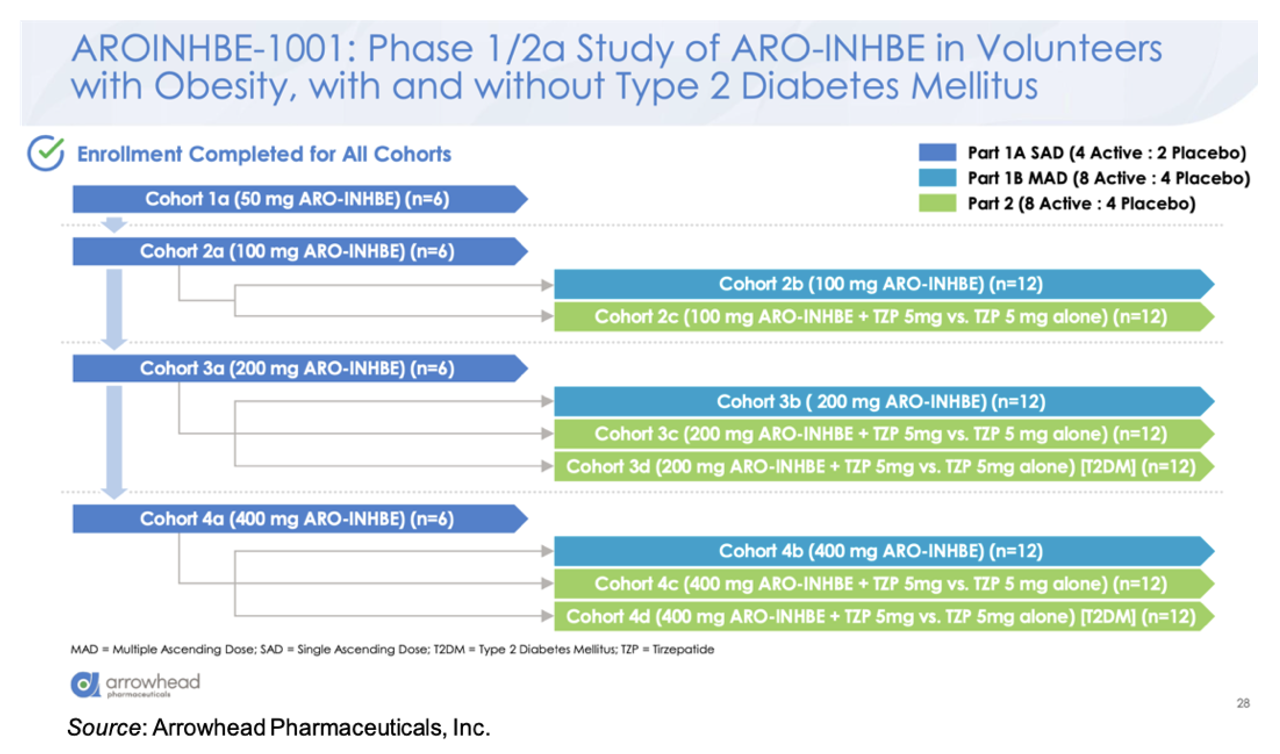
ARO-INHBE is being evaluated in the AROINHBE-1001 study. This is a Phase 1/2a clinical trial in obese volunteers both with and without type 2 diabetes (T2D). An overview of the trial is given below. Part 1 of the trial is designed to test single and multiple doses of the drug as a monotherapy. Part 2 of the study is designed to assess ARO-INHBE in combination with tirzepatide. Enrollment has been completed for all cohorts. The primary endpoints for the study are safety and pharmacokinetics, with exploratory endpoints evaluating serum Activin E levels, weight change, body composition, liver fat content (MRI-PDFF), fasting lipids, and glycemic control parameters.
There was a dose response in the knockdown of serum Activin E, with a mean maximum reduction of -85% at the 400 mg dose. The reduction continued for three months following a single dose. The single dose of ARO-INHBE resulted in reductions in a number of important parameters at Week 16, including visceral adipose tissue (-9.9%) and liver fat content (-37.8%), while simultaneously causing an increase in lean tissue mass (+3.6%) and a decrease in muscle fat infiltration (-0.7%). Repeated doses of ARO-INHBE further improved visceral fat reduction by up to -15.6%.
For the combination cohort (tirzepatide 5 mg weekly + ARO-INHBE 200 mg, ARO-INHBE 400 mg, or placebo at Week 0 and Week 4), the company presented the data for the T2D cohort and will share the data for the non-diabetic cohort at a later date. The combination therapy decreased serum Activin E levels up to 84% in the T2D cohort, while no decrease in Activin E was seen in the tirzepatide monotherapy cohort. At Week 16, the 400 mg ARO-INHBE + 5 mg tirzepatide group showed a weight change of -9.4% compared to -4.8% for the placebo + 5 mg tirzepatide cohort.
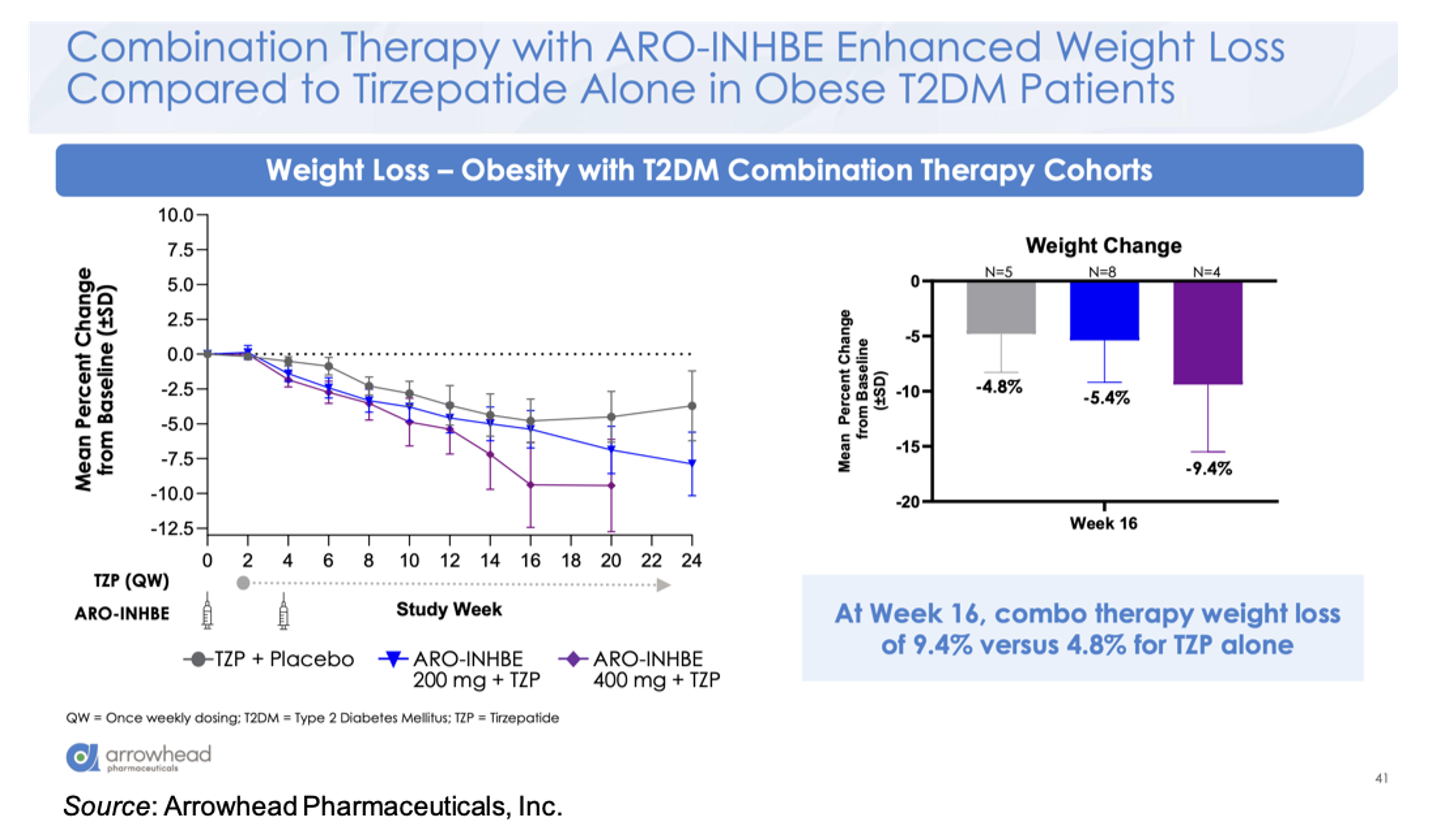
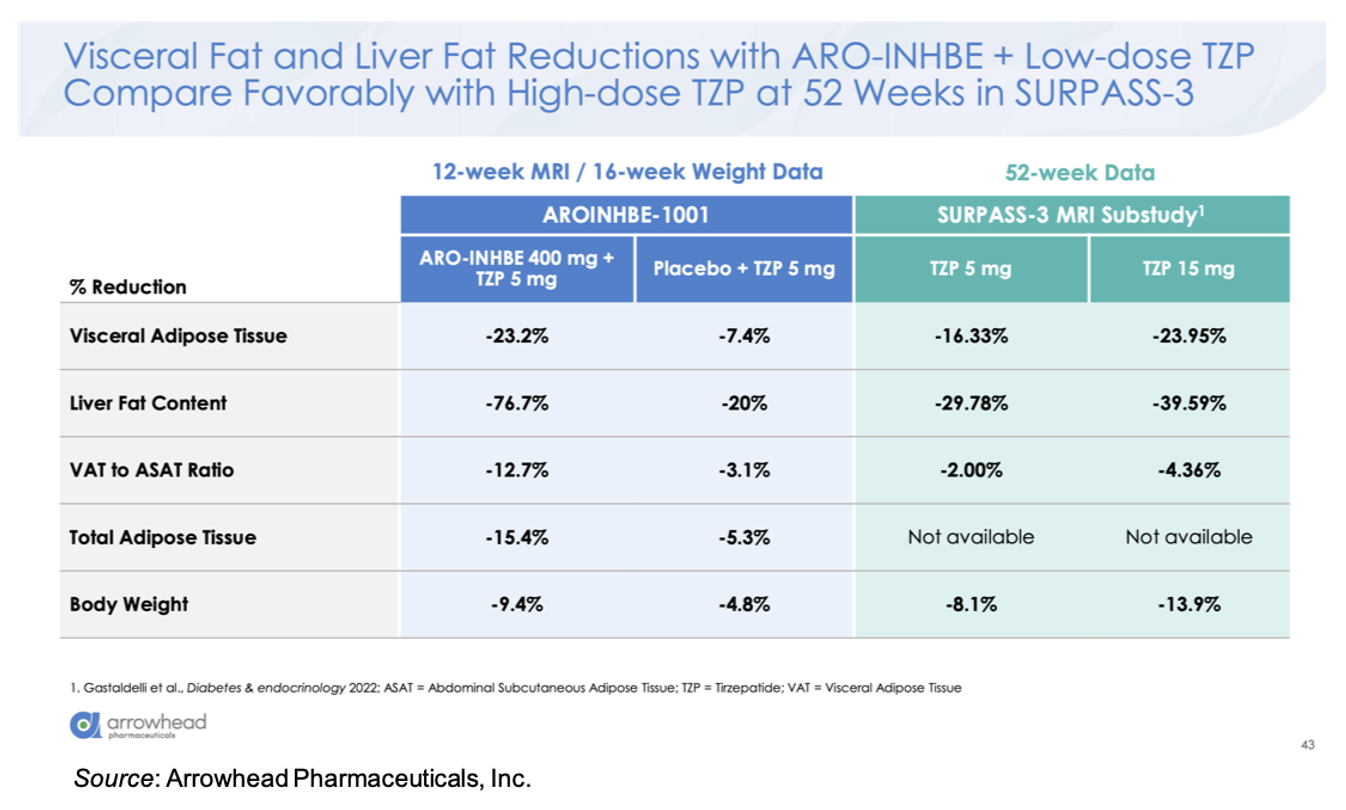
Compared to 5 mg tirzepatide alone, there was an approximately three-fold enhancement in loss of visceral adipose tissue, total adipose tissue, and liver fat content in the ARO-INHBE 400 mg + 5 mg tirzepatide. Interestingly, these reductions at Week 12 (MRI data)/Week 16 (Weight data) are on track to meet or exceed the Week 52 data seen in the SURPASS-3 MRI-substudy (Gastaldelli et al., 2022).
ARO-INHBE was well tolerated in the study as both a monotherapy and in combination with tirzepatide. Most of the treatment emergent adverse events (TEAEs) were mild in intensity and there were no TEAEs that resulted in discontinuation from the study or the study drug. The frequency of GI adverse events was similar between the tirzepatide monotherapy and the combination cohorts. Lastly, there were no clinically significant adverse laboratory trends including liver enzymes, glycemic indices, or lipid parameters.
ARO-ALK7
ARO-ALK7 is being evaluated in the Phase 1/2a AROALK7-1001 study. This clinical trial is set up almost exactly like the INHBE trial. Obese volunteers are being enrolled into Part 1 of the trial to receive single ascending doses (that part of the trial is fully enrolled), and that will be followed by multiple ascending dose cohorts for both monotherapy and combination therapy in both diabetic and non-diabetic patients. One key difference from the INHBE trial is that adipose biopsies are being used to measure knockdown of ALK7 mRNA since there is no serum biomarker available like there is for INHBE. In addition, the multiple doses of ARO-ALK7 are taking place three months apart, which is in contrast to ARO-INHBE that is dosed on Day 0 and Day 29. This is due to the long duration of activity seen in non-human primates.
The following figure shows the mean change from baseline in ALK7 mRNA for the different doses, with a mean decrease of -88% in the 200 mg cohort. This is a very important observation as it validates the ability of the platform to target gene silencing in adipocytes.
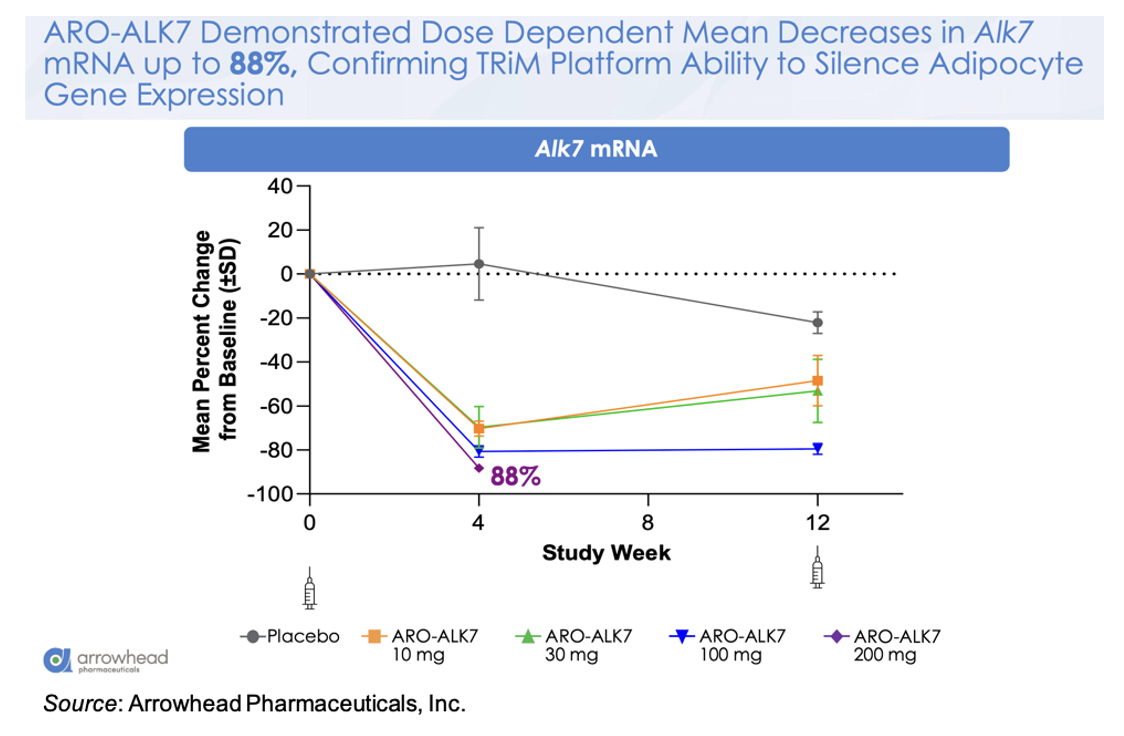
This knockdown of ALK7 mRNA translates to a placebo-adjusted dose-dependent reduction in visceral fat at Week 8 of up to 14.1% after a single dose of ARO-ALK7. These very early data may be alluding to greater activity in ARO-ALK7 compared to ARO-INHBE, however additional data will need to be collected to support that observation. Preliminary safety data also shows that ARO-ALK7 has been well tolerated as a monotherapy, with most TEAEs being mild in severity and no TEAEs leading to study or study drug discontinuation.
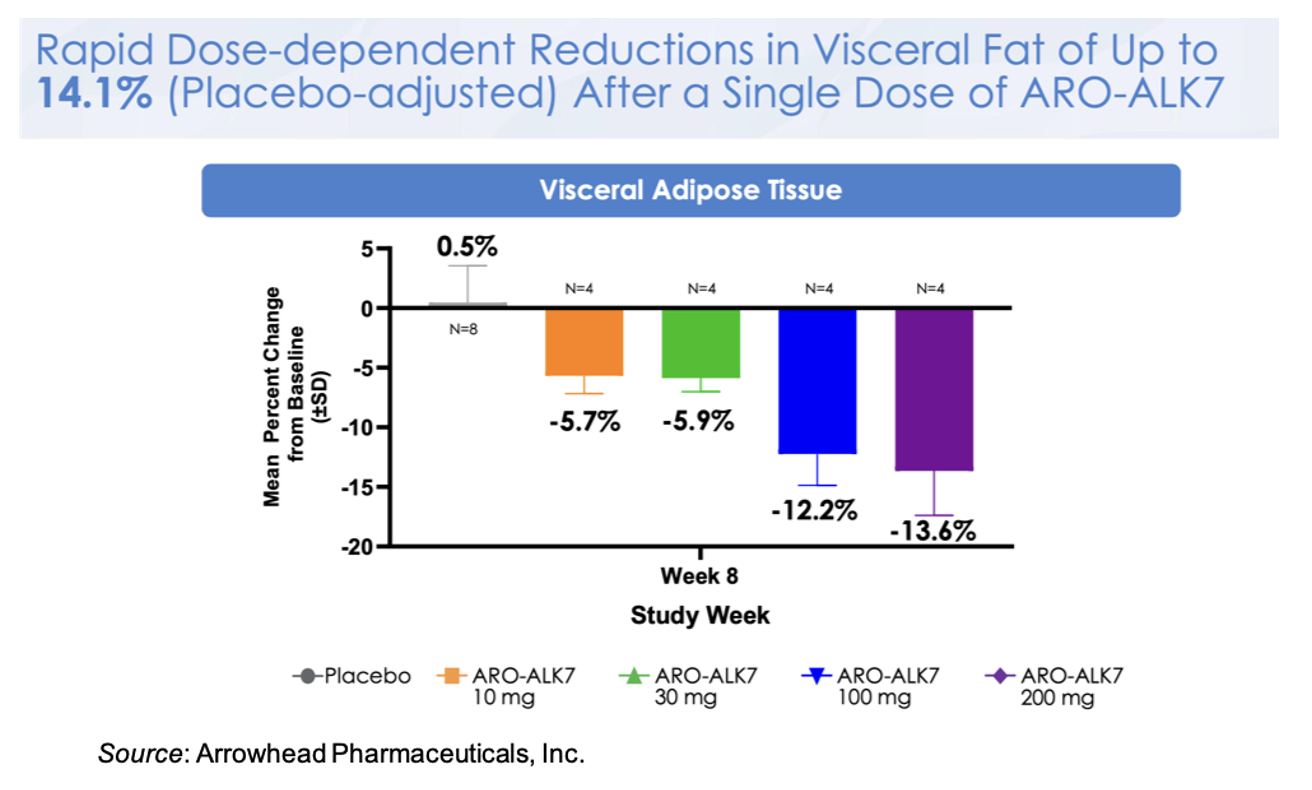
Next Steps
Arrowhead will be increasing the number of patients in these studies to increase their power along with extending the follow-up time to better understand the drug’s durability and activity out to one year. In addition, a monotherapy cohort in obese diabetic patients will be initiated along with additional combination cohorts with other GLP-1s. The company is also moving as quickly as possible to Phase 2b studies and not waiting on final results from the ongoing Phase 1/2a studies. The Phase 2b studies will be combination studies with tirzepatide and other GLP-1s in obese diabetic patients along with studies aimed at the use of ARO-INHBE and ARO-ALK7 as maintenance therapies following the cessation of GLP-1 therapy. Lastly, Arrowhead will be expanding the obesity pipeline to include additional liver and adipose targets, dimers targeting two adipose targets, dimers targeting two liver targets, and leveraging the CNS platform to address targets in the brain.
Early Comparison to Wave Life Sciences WVE-007
Wave Life Sciences, Ltd. (WVE) is developing WVE-007 (INHBE GalNAc-siRNA) as a treatment for obesity. Just like ARO-INHBE, it is designed to silence the INHBE gene. Wave is currently evaluating WVE-007 in the Phase 1 INLIGHT trial and recently announced interim data from the lowest dose cohort. The data showed that a single 240 mg dose of WVE-007 lead to a placebo-adjusted 9.2% reduction in visceral fat, a 4.0% reduction in total body fat, and a 0.9% increase in lean mass as measured by DEXA scan at Day 85. A table with the WVE-007 and ARO-INHBE data is presented below.
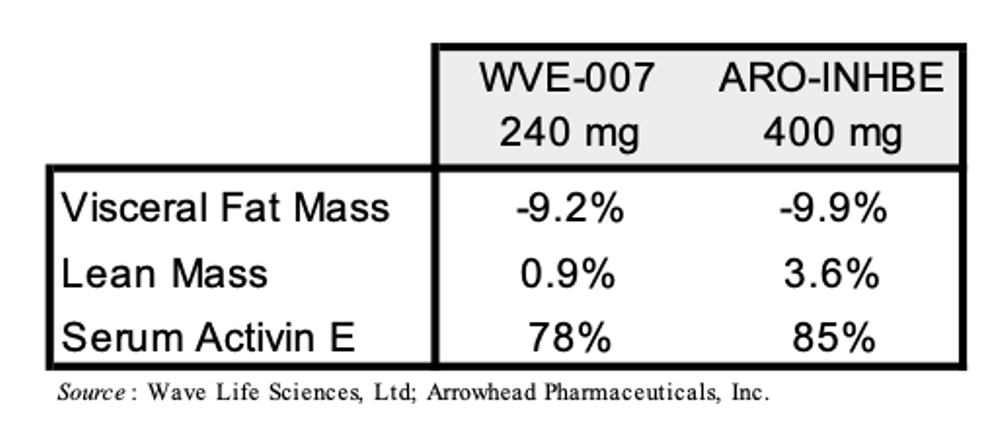
A few caveats to the above comparison are warranted: 1) Arrowhead’s trial (NCT00670538) allows for patients with BMI’s between 30-50 kg/m3 while the INLIGHT trial (NCT06842186) only allows for patients with BMIs of 28-35 kg/m3; 2) Arrowhead’s trial lists as part of the inclusion criteria “Willing, able and motivated to comply with all study assessments and adhere to the protocol schedule, including adherence to a stable diet and exercise routine for the duration of the study” while there is no diet or exercise modifications mentioned for the INLIGHT trial; 3) the data from Arrowhead’s trial above is from 16 weeks but the data from the INLIGHT trial is from 12 weeks. Investors should keep these points in mind as Wave and Arrowhead announce additional data from these trials in the future.
Conclusion
We’re very encouraged by the early data presented by Arrowhead for ARO-INHBE and ARO-ALK7. We anticipate additional updates for both of these programs throughout 2026 that will include the initiation of Phase 2b trials as well as additional data from the Phase 1/2a trials. It’s still too early to know what regulatory pathway these compounds will take; however, the company has indicated that, at this point, it intends to continue development of both drugs. Based on the data thus far, we have modestly increased their probabilities of approval, which has resulted in a slight increase in our valuation to $78 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.