By David Bautz, PhD
NASDAQ:ARWR
READ THE FULL ARWR RESEARCH REPORT
Business Update
First Commercial Sales of REDEMPLO®
Following its approval in November 2025, Arrowhead Pharmaceuticals, Inc. (NASDAQ:ARWR) recently announced encouraging news from the early commercial launch of REDEMPLO (plozasiran) as a treatment for adults suffering from familial chylomicronemia syndrome (FCS). Even with the Thanksgiving, Christmas, and New Year’s holidays, the company reported that over 100 prescriptions for REDEMPLO have been received from a diverse prescriber base that is geographically balanced across the U.S. The early patients fall into one of three cohorts: patients transitioning from the Expanded Access Program, patients naïve to the APOC3 class, and patients switching from olezarsen. The company did not disclose revenues for REDEMPLO, but will do so when they become a meaningful driver of the financials.
Arrowhead also recently announced that REDEMPLO was approved by Health Canada and the Chinese National Medical Products Administration. The drug will be available in Canada later this year, and we anticipate it being marketed independently by Arrowhead like in the U.S. In Greater China, REDEMPLO will be marketed by Sanofi. Pending regulatory review and approval, the company anticipates potentially launching REDEMPLO later this year in select EU countries and in the U.K.
ARO-DIMER-PA Trial Underway
Arrowhead recently announced the first patients were dosed in a Phase 1/2 clinical trial of ARO-DIMER-PA, which is designed to prevent atherosclerotic cardiovascular disease (ASCVD) due to mixed hyperlipidemia by silencing the expression of two genes: proprotein convertase subtilisin/kexin type 9 (PCSK9) and apolipoprotein C3 (APOC3). This is the first RNAi clinical candidate to target two genes simultaneously in one molecule. Mixed hyperlipidemia is characterized by elevated low-density lipoprotein cholesterol (LDL-C) and triglycerides (TGs) and is a major risk factor for ASCVD. Preclinical data for ARO-DIMER-PA were presented at the 2025 National Lipid Association Annual Scientific Sessions in May 2025. A copy of the poster presentation can be accessed here. The results of those studies showed that ARO-DIMER-PA potently lowered serum PCSK9 and APOC3 while also decreasing levels of non-HDL cholesterol, LDL-C, and TGs in dyslipidemic nonhuman primates. We anticipate interim data from the Phase 1/2 trial in the second half of 2026.
ARO-MAPT Phase 1/2 Trial Underway
Arrowhead recently initiated the Phase 1/2 clinical trial of ARO-MAPT, which is being developed for the treatment of tauopathies, including Alzheimer’s disease. The trial will include both healthy volunteers and Alzheimer’s patients. ARO-MAPT is the first therapy developed by Arrowhead that can penetrate the blood-brain barrier to enable knockdown of target genes in the central nervous system (CNS). Tau protein is encoded by the MAPT gene and is highly expressed in neurons, where it stabilizes microtubules in axons. Hyperphosphorylation of tau protein promotes neurofibrillary tangles, which are correlated with neurodegeneration. In Alzheimer’s disease, tau neurofibrillary tangles are predictive of cognitive decline, and currently available anti-amyloid therapies only result in minimal tau reduction. At the 2025 RNA Leaders USA Congress, Arrowhead presented preclinical data that showed deep knockdown of MAPT mRNA throughout the CNS following subcutaneous administration of ARO-MAPT in nonhuman primates. This reduction in MAPT mRNA translated into long lasting reduction in tau protein, with pharmacokinetic (PK) data showing the potential for once monthly or once quarterly dosing. We anticipate interim data from the healthy volunteer portion of the study should be available in 2026 and from Alzheimer’s patients in 2027.
Encouraging Early Data for Obesity Programs
In January 2026, Arrowhead announced preliminary results for the company’s investigational treatments for obesity, ARO-INHBE and ARO-ALK7. The company also held a webinar to discuss the results. The slides and the webinar can be accessed here. The data showed that ARO-INHBE, both as a monotherapy and in combination with tirzepatide, led to reductions in visceral fat, total fat, and liver fat. These were early data, thus the number of patients treated so far is small, however the early signals look promising for each of the assets.
With the success of the different GLP-1 medications already on the market, some may question if there is a need for additional obesity therapeutics. Dr. Carel le Roux presented on the unmet need in obesity treatment. Much like other chronic diseases have targets that doctors encourage their patients to achieve (e.g., <6.5% for HbA1c, <130/80 for blood pressure, etc.), Dr. le Roux and colleagues proposed obesity targets that were back-tested and found that a waist-to-height ratio (WHtR) of <0.53 and/or a BMI <27 kg/m3 after weight loss were found to decrease the risk of T2D, hip/knee osteoarthritis, and atherosclerotic cardiovascular disease (ASCVD). Interestingly, while both semaglutide and tirzepatide cause significant weight loss, based on results from the SURMOUNT-5 study (Aronne et al., 2025), only 14.2% (semaglutide) and 23.1% (tirzepatide) of patients from that study hit the aforementioned targets. This is important, as >50% of patients that achieved WHtR of <0.53 and/or BMI <27 kg/m3 had normalization of cardiometabolic parameters as well.
Additionally, even when only examining weight loss, diabetic patients consistently underperform non-diabetic patients, thus suggesting that those patients are achieving the WHtR and BMI targets at an even lower rate. This means that those individuals are going to need additional options to help them achieve the targets. While there are a very large number of MOAs being tested in the clinic, targeting the Activin E-ALK7 pathway represents a novel means to target adipose tissue.
ARO-INHBE targets the INHBE gene that encodes activin E, which is a ligand for ALK7. ARO-ALK7 is designed to reduce the ALK7 receptor, which binds activin E, and is a TGF-β superfamily member that is expressed in adipocytes.
Support for targeting INHBE derives from two papers that came out in 2022 that described loss of function mutations in INHBE that were associated with favorable fat distribution. Deaton et al. reported a genome-wide association study (GWAS) from 362,679 individuals that showed a predicted loss of function variant in INHBE associated with a lower waist-to-hip ratio adjusted for BMI (WHRadjBMI), which they used as a surrogate for abdominal fat that is causally linked to type 2 diabetes and coronary heart disease (Deaton et al., 2022). Akbari et al. performed a GWAS in 618,375 individuals and identified an association with favorable fat distribution, favorable metabolic profile, and protection from type 2 diabetes for heterozygous protein-truncating mutations in INHBE (Akbari et al., 2022). Arrowhead has conducted studies of Inhbe silencing in mouse obesity models with results showing reduced weight gain compared to controls. Importantly, the difference in weight gain was primarily due to changes in fat mass with no difference in lean mass.
Similar to the data supporting targeting INHBE, a GWAS study in 2019 showed that four variants in the ACVR1C gene (which encodes ALK7) were associated with reduced percent abdominal fat in DEXA imaging, a lower WHRadjBMI, and a decreased risk of developing type 2 diabetes (Emdin et al., 2019).
ARO-INHBE
ARO-INHBE is being evaluated in the AROINHBE-1001 study. This is a Phase 1/2a clinical trial in obese volunteers both with and without type 2 diabetes (T2D). An overview of the trial is given below. Part 1 of the trial is designed to test single and multiple doses of the drug as a monotherapy. Part 2 of the study is designed to assess ARO-INHBE in combination with tirzepatide. Enrollment has been completed for all cohorts. The primary endpoints for the study are safety and pharmacokinetics, with exploratory endpoints evaluating serum Activin E levels, weight change, body composition, liver fat content (MRI-PDFF), fasting lipids, and glycemic control parameters.
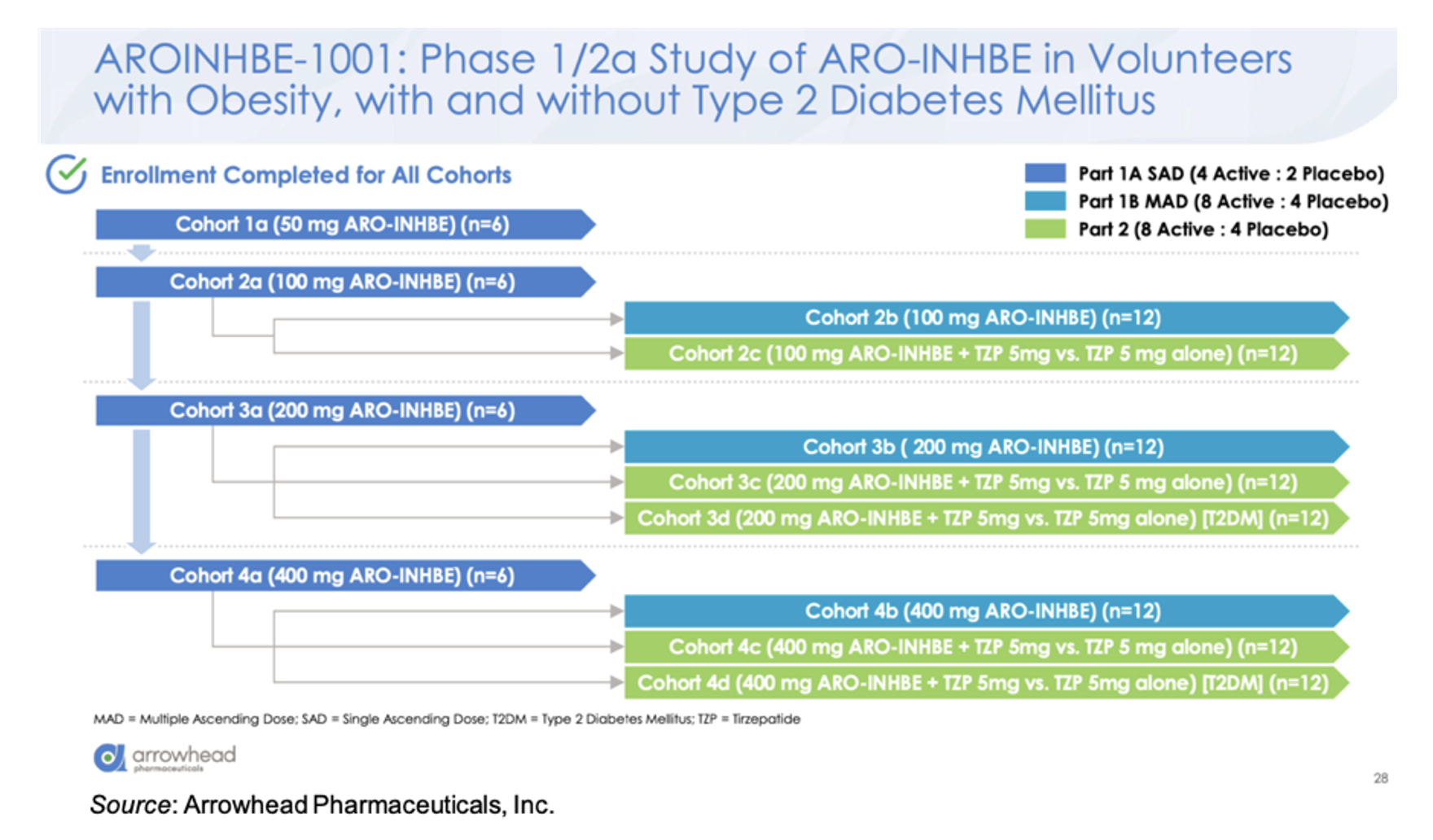
There was a dose response in the knockdown of serum Activin E, with a mean maximum reduction of -85% at the 400 mg dose. The reduction continued for three months following a single dose. The single dose of ARO-INHBE resulted in reductions in a number of important parameters at Week 16, including visceral adipose tissue (-9.9%) and liver fat content (-37.8%), while simultaneously causing an increase in lean tissue mass (+3.6%) and a decrease in muscle fat infiltration (-0.7%). Repeated doses of ARO-INHBE further improved visceral fat reduction by up to -15.6%.
For the combination cohort (tirzepatide 5 mg weekly + ARO-INHBE 200 mg, ARO-INHBE 400 mg, or placebo at Week 0 and Week 4), the company presented the data for the T2D cohort and will share the data for the non-diabetic cohort at a later date. The combination therapy decreased serum Activin E levels up to 84% in the T2D cohort, while no decrease in Activin E was seen in the tirzepatide monotherapy cohort. At Week 16, the 400 mg ARO-INHBE + 5 mg tirzepatide group showed a weight change of -9.4% compared to -4.8% for the placebo + 5 mg tirzepatide cohort.
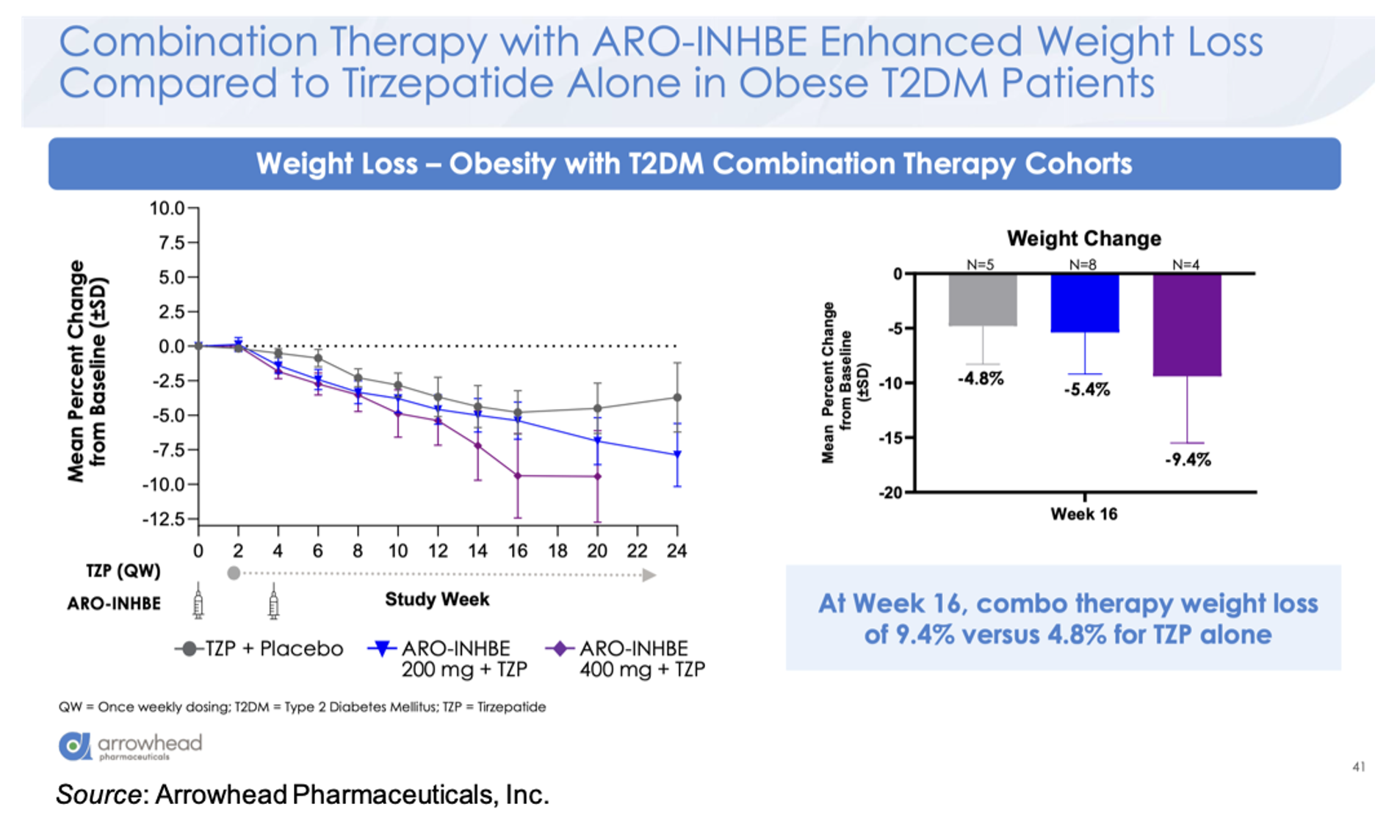
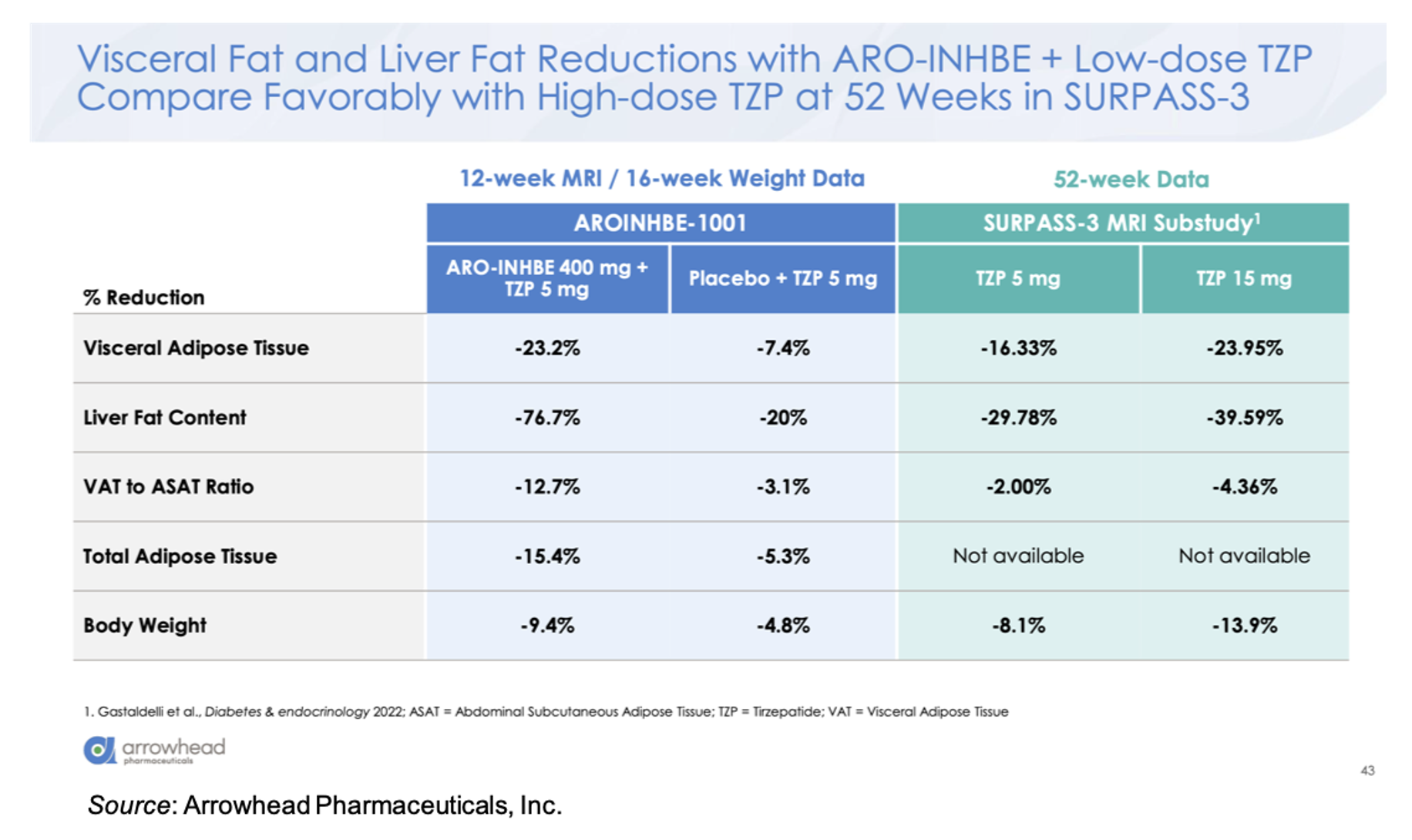
Compared to 5 mg tirzepatide alone, there was an approximately threefold enhancement in loss of visceral adipose tissue, total adipose tissue, and liver fat content in the ARO-INHBE 400 mg + 5 mg tirzepatide. Interestingly, these reductions at Week 12 (MRI data)/Week 16 (Weight data) are on track to meet or exceed the Week 52 data seen in the SURPASS-3 MRI-substudy (Gastaldelli et al., 2022).
ARO-INHBE was well tolerated in the study as both a monotherapy and in combination with tirzepatide. Most of the treatment emergent adverse events (TEAEs) were mild in intensity, and there were no TEAEs that resulted in discontinuation from the study or the study drug. The frequency of GI adverse events was similar between the tirzepatide monotherapy and the combination cohorts. Lastly, there were no clinically significant adverse laboratory trends, including liver enzymes, glycemic indices, or lipid parameters.
ARO-ALK7
ARO-ALK7 is being evaluated in the Phase 1/2a AROALK7-1001 study. This clinical trial is set up almost exactly like the INHBE trial. Obese volunteers are being enrolled into Part 1 of the trial to receive single ascending doses (that part of the trial is fully enrolled), and that will be followed by multiple ascending dose cohorts for both monotherapy and combination therapy in both diabetic and non-diabetic patients. One key difference from the INHBE trial is that adipose biopsies are being used to measure knockdown of ALK7 mRNA, since there is no serum biomarker available like there is for INHBE. In addition, the multiple doses of ARO-ALK7 are taking place three months apart, which is in contrast to ARO-INHBE that is dosed on Day 0 and Day 29. This is due to the long duration of activity seen in non-human primates.
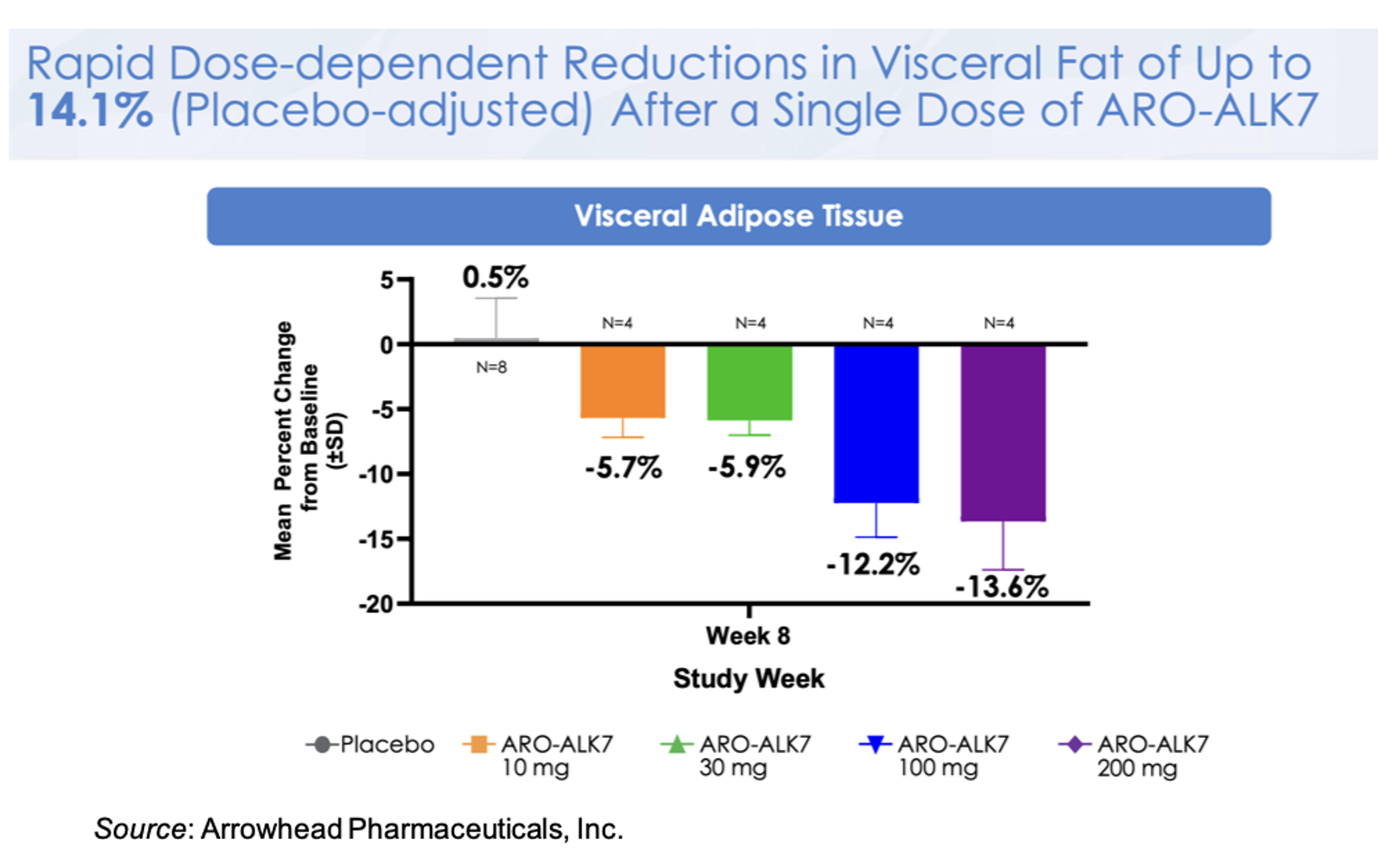
The following figure shows the mean change from baseline in ALK7 mRNA for the different doses, with a mean decrease of -88% in the 200 mg cohort. This is a very important observation as it validates the ability of the platform to target gene silencing in adipocytes.
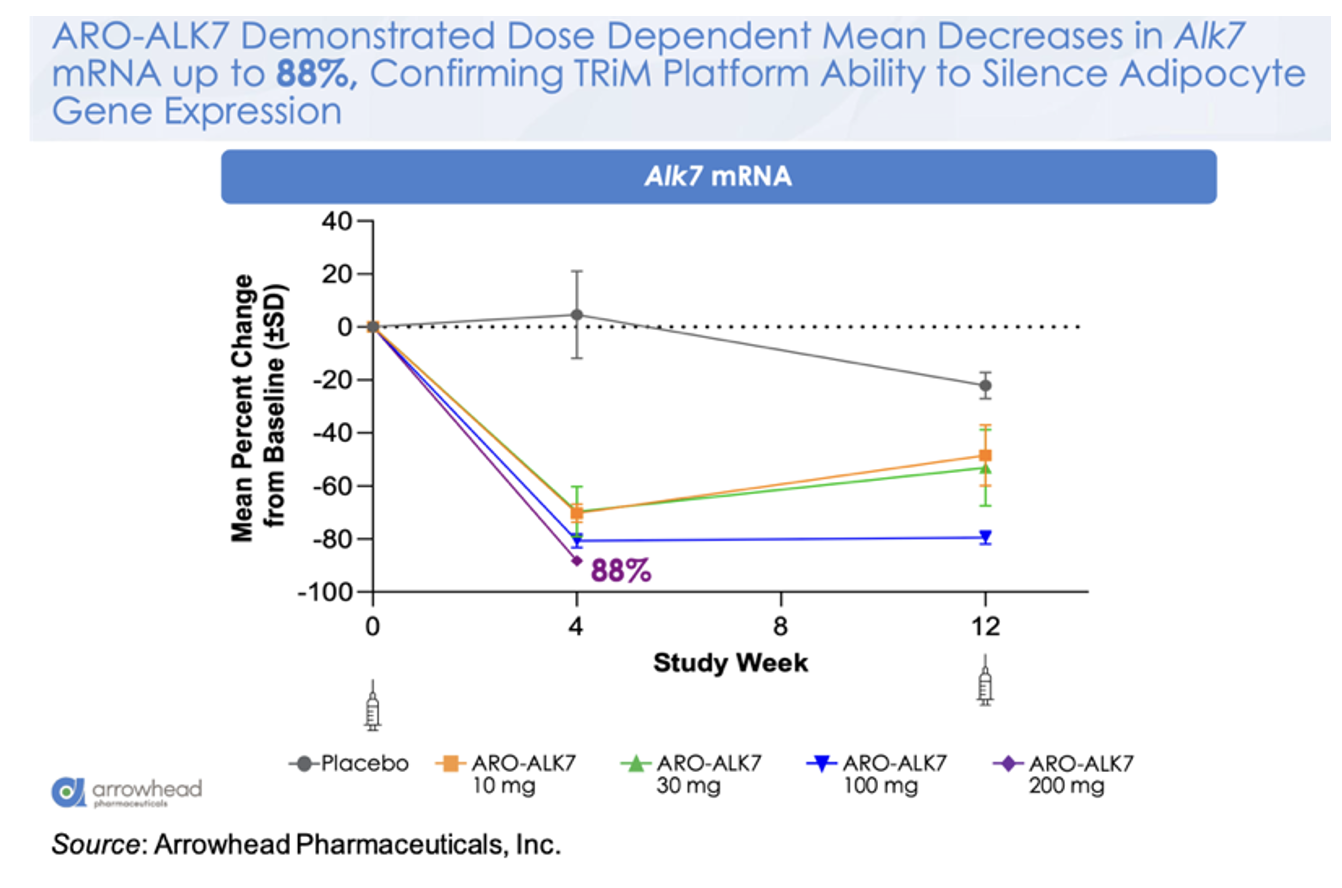
This knockdown of ALK7 mRNA translates to a placebo-adjusted dose-dependent reduction in visceral fat at Week 8 of up to 14.1% after a single dose of ARO-ALK7. These very early data may be alluding to greater activity in ARO-ALK7 compared to ARO-INHBE, however additional data will need to be collected to support that observation. Preliminary safety data also shows that ARO-ALK7 has been well tolerated as a monotherapy, with most TEAEs being mild in severity and no TEAEs leading to study or study drug discontinuation.
Next Steps
Arrowhead will be increasing the number of patients in these studies to increase their power along with extending the follow-up time to better understand the drug’s durability and activity out to one year. In addition, a monotherapy cohort in obese diabetic patients will be initiated along with additional combination cohorts with other GLP-1s. The company is also moving as quickly as possible to Phase 2b studies and not waiting on final results from the ongoing Phase 1/2a studies. The Phase 2b studies will be combination studies with tirzepatide and other GLP-1s in obese diabetic patients, along with studies aimed at the use of ARO-INHBE and ARO-ALK7 as maintenance therapies following the cessation of GLP-1 therapy. Lastly, Arrowhead will be expanding the obesity pipeline to include additional liver and adipose targets, dimers targeting two adipose targets, dimers targeting two liver targets, and leveraging the CNS platform to address targets in the brain.
Early Comparison to Wave Life Sciences WVE-007
Wave Life Sciences, Ltd. (WVE) is developing WVE-007 (INHBE GalNAc-siRNA) as a treatment for obesity. Just like ARO-INHBE, it is designed to silence the INHBE gene. Wave is currently evaluating WVE-007 in the Phase 1 INLIGHT trial and recently announced interim data from the lowest dose cohort. The data showed that a single 240 mg dose of WVE-007 lead to a placebo-adjusted 9.2% reduction in visceral fat, a 4.0% reduction in total body fat, and a 0.9% increase in lean mass as measured by DEXA scan at Day 85. A table with the WVE-007 and ARO-INHBE data is presented below.
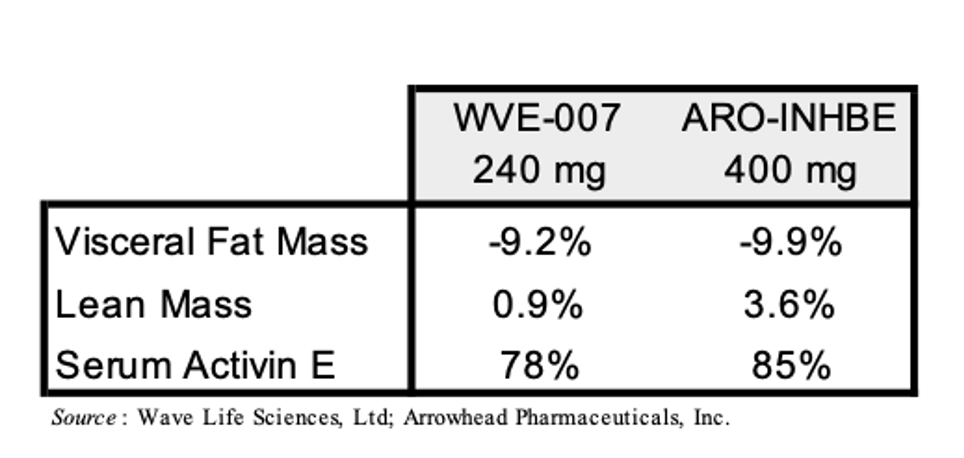
A few caveats to the above comparison are warranted: 1) Arrowhead’s trial (NCT00670538) allows for patients with BMI’s between 30-50 kg/m3 while the INLIGHT trial (NCT06842186) only allows for patients with BMI’s of 28-35 kg/m3; 2) Arrowhead’s trial lists as part of the inclusion criteria “Willing, able and motivated to comply with all study assessments and adhere to the protocol schedule, including adherence to a stable diet and exercise routine for the duration of the study” while there is no diet or exercise modifications mentioned for the INLIGHT trial; 3) the data from Arrowhead’s trial above is from 16 weeks but the data from the INLIGHT trial is from 12 weeks. Investors should keep these points in mind as Wave and Arrowhead announce additional data from these trials in the future.
Financial Update
On February 5, 2026, Arrowhead announced financial results for the first quarter of fiscal year 2026 that ended December 31, 2025. The company reported revenue of approximately $264 million for the first quarter of fiscal year 2026 compared to approximately $2.5 million for the first quarter of fiscal year 2025. The revenue was primarily driven by revenue recognition associated with Sarepta ($229.5 million) and Novartis ($34.2 million) license agreements.
R&D expenses for the first quarter of fiscal year 2026 were $177.2 million compared to $137.0 million for the first quarter of fiscal year 2025. The increase was primarily driven by increased candidate costs (clinical trial costs, manufacturing, and toxicity study costs), R&D discovery costs (expansion into novel therapeutic areas and tissue types), and salaries. G&A expenses for the first quarter of 2026 were $46.0 million compared to $26.9 million for the first quarter of fiscal year 2025. The increase was primarily due to salaries, professional costs (product launch costs including data analytics, marketing, and commercial launch support), and non-cash, stock-based compensation.
Arrowhead exited the first quarter of fiscal year 2026 with approximately $916 million in cash, cash equivalents, and investments. The reported cash balance does not include the $200 million that the company earned for the DM1 second milestone from Sarepta, which was received in January 2026. Nor does it include the $50 million anniversary payment that is expected to be received from Sarepta on or before February 10, 2026. Lastly, the cash balance of $916 million does not include the financing transaction that the company undertook in January 2026, consisting of a concurrent offering of convertible senior notes ($625 million of 0.00% convertible senior notes due 2032) and common stock (3,100,776 shares at a price of $64.50), along with associated capped call transactions. The initial conversion rate for the convertible senior notes is 11.4844 share of common stock per $1,000 principal of notes, which represents an initial conversion price of approximately $87.07 per share of common stock. The capped call transactions will cover the number of shares of Arrowhead’s common stock underlying the notes to offset any potential dilution. The cap price of the capped call transactions will initially be approximately $119.33 per share. If the market price of Arrowhead’s common stock exceeds the cap price of the capped call transactions, there would be dilution. The company estimates that at any share price below $119, the estimated total cost of capital is approximately 1.5%.
Conclusion
The early launch data for REDEMPLO is encouraging and we look forward to additional updates throughout 2026 on the progress of the launch. We anticipate multiple data readouts throughout the year, including for the SHASTA-3 and SHASTA-4 studies of plozasiran in SHTG in the third quarter of 2026, ARO-DIMER-PA data in the second half of 2026, early ARO-MAPT data, and additional data presentations for ARO-INHBE and ARO-ALK7. The recent financing was done on extremely favorable terms for the company, which we believe is indicative of strong institutional support and gives Arrowhead substantial flexibility to support both the current and future clinical and commercialization plans. Our valuation remains at $78 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.