By John Vandermosten, CFA
NASDAQ:CRDF
READ THE FULL CRDF RESEARCH REPORT
On January 27th, 2026, Cardiff Oncology, Inc. (NASDAQ:CRDF) announced that its CEO, Dr. Mark Erlander, and CFO, James Levine, stepped down from their roles. The new chief executive officer is Dr. Mani Mohindru, who has been on Cardiff’s board since 2021; the lead finance role was assumed by Brigitte Lindsay, who was appointed as Chief Accounting Officer. Along with the appointment of new executives, the company presented select data from its Phase II trial in onvansertib in first line RAS-mutated metastatic colorectal cancer (mCRC). Results from the CRDF-004 trial supported the selection of the 30 mg onvansertib dose for the anticipated Phase III program in first-line RAS-mutated mCRC. Results also validate previously reported results from the Phase II second-line mCRC trial, where bevacizumab-naïve patients demonstrated a clinical benefit from the use of onvansertib. Along with the data readout, company management explained the data and answered analyst questions in a conference call.
Executive Leadership Change
Mani Mohindru, Ph.D., was appointed interim CEO following the departure of Dr. Mark Erlander. She has previously served as CEO of Novasanta, CEO of CereXis, and other financial and strategic roles at other oncology and pain companies. Prior to her executive roles, she was a biotechnology analyst at UBS, Credit Suisse, and ThinkEquity. Dr. Mohindru also serves in several board of director roles, including that of Cardiff Oncology, where she has been a director since 2021. She received her Ph.D. in Neurosciences from Northwestern University and her master’s in biotechnology and BS in Human Biology from the All-India Institute of Medical Sciences, India.
Brigitte Lindsay was appointed as Cardiff’s Chief Accounting Officer in January 2026 and has been with the company for 14 years. Prior roles include controller for AviaraDx and manager of financial reporting and analysis for Carl Zeiss Meditec. Ms. Lindsay received her Diplom Betriebswirt from the Verwaltungs und Wirtschaftsakademie in Munich, Germany.
Onvansertib Phase II Readout
In a January 27th press release, Cardiff reported select data from its CRDF-004 Phase II trial of onvansertib, demonstrating dose-dependent response rates for overall survival and durability as measured by progression-free survival (PFS) in patients with RAS-mutated mCRC. Data released includes overall response rates (ORR), median PFS, and the associated Hazard Ratio (HR). Final data and registrational plans are planned to be released in 1H:26.
CRDF-004 Background
Cardiff’s lead program is evaluating onvansertib in the treatment of first line, RAS-mutated, mCRC. CRDF-004 trial’s official title is: Study of Onvansertib in Combination with FOLFIRI and Bevacizumab or FOLFOX and Bevacizumab Versus FOLFIRI and Bevacizumab or FOLFOX and Bevacizumab for First-Line Treatment of Metastatic Colorectal Cancer in Adult Participants with a KRAS or NRAS Mutation. It is listed under the designator NCT06106308 on clinicaltrials.gov. It is a Phase II study that enrolled 110 subjects at over 40 sites around the United States.
Trial Design
CRDF-004 was designed to find the lowest effective dose and to assess the safety, efficacy, and pharmacokinetic profile of onvansertib. The trial enrolled first-line mCRC patients that are KRAS or NRAS positive with unresectable tumors and no prior treatment with bevacizumab (bev). It enrolled six randomization arms of onvansertib combined with bevacizumab and FOLFIRI+bev[1] or FOLFOX+bev[2]. Control arms were standard of care,[3] FOLFIRI+bev, and FOLFOX+bev. Two arms evaluated 20 mg of onvansertib with one or the other standard of care arms, and two arms evaluated 30 mg of onvansertib with each of the two standard-of-care arms. The primary endpoint is overall response rate (ORR). Secondary endpoints included duration of response (DoR), measurement of adverse events, overall survival (OS), and progression-free survival (PFS), as well as several pharmacokinetic and pharmacodynamic metrics.
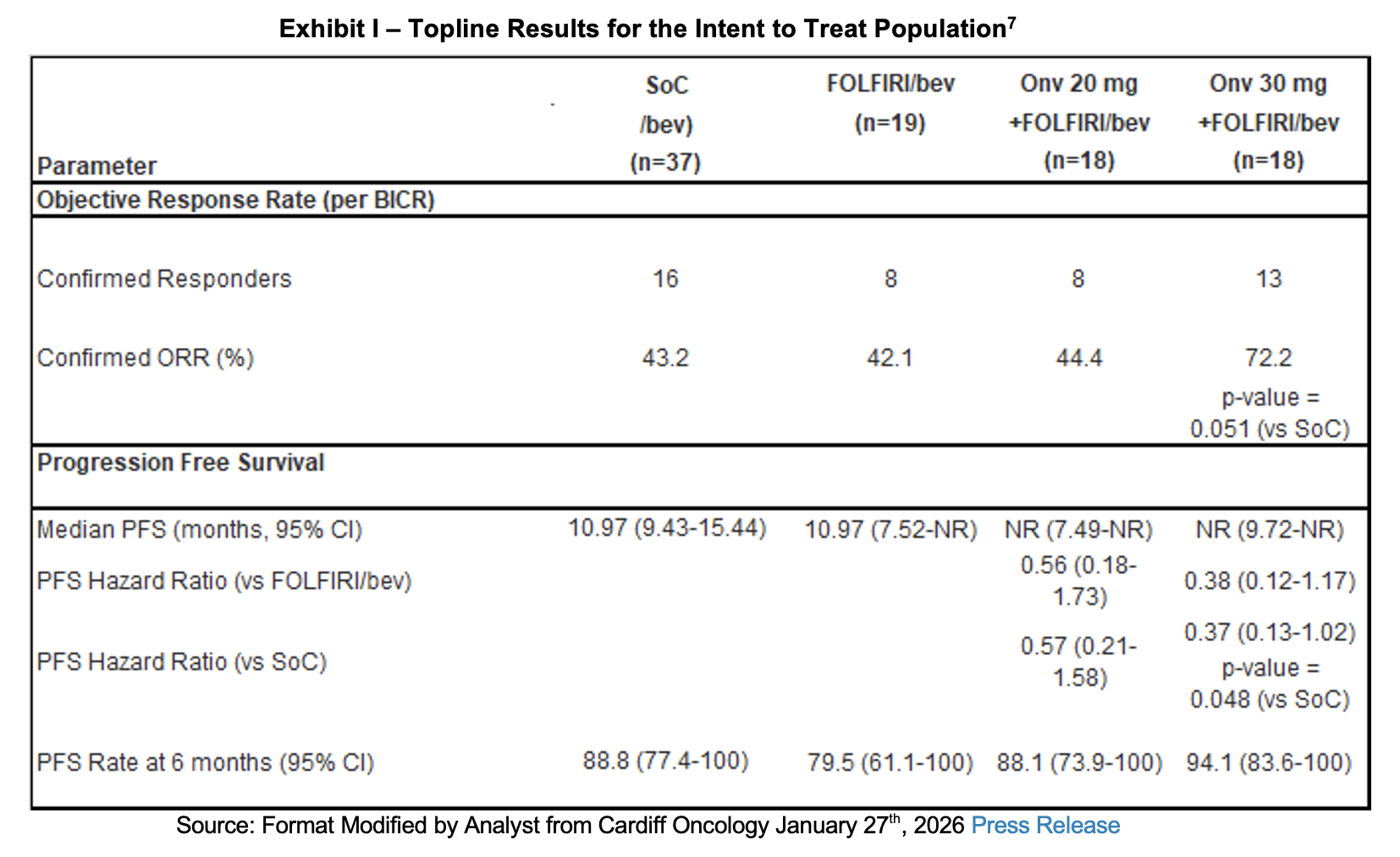
Results
The data released shows dose dependent benefits across multiple efficacy measures, especially those in the active arm whose treatment included FOLFIRI. As of the data cut-off on January 22nd, 2026, the onvansertib 30 mg arm generated an overall response rate (ORR) of 72.2% compared to the control arm’s 43.2%. The difference was associated with a p-value of 0.051.[4] Median PFS was not reached for either the 20 mg or 30 mg onvansertib arms and is about 11 months in the SoC arm. The PFS Hazard Ratio[5] was 0.37 compared with SoC with a p-value of 0.048.[6] Progression-free survival at six months was 94.1% for the 30 mg onvansertib arm and 88.8% in the SoC arm. The objective of the Phase II study was to identify a dose for the registrational trial and provide additional safety and efficacy data; however, the data is generated on a small number of patients, and it is not yet mature. Note that comparisons do not include data from the onvansertib + FOLFOX arms.
Management expects to meet with regulators in the near term, sharing this and additional data in an effort to design the Phase III registrational trial. We anticipate this will take place during the first half of 2026.
Safety Summary
Onvansertib in combination with both chemotherapy and bevacizumab was well-tolerated. There were no major or unexpected toxicities observed and no additive adverse events. Grade 3 or higher adverse events were infrequent, with neutropenia being the most common treatment-emergent adverse event across both the onvansertib combination and standard of care arms.
Milestones
- Roger Sidhu appointed CMO – June 2025
- Interim update on CRDF-004 trial – July 2025
- Poster presentation of investigator-sponsored data in CMML at ASH – December 2025
- Presentation at Sidoti’s investor conference – December 2025
- Mani Mohindru appointed interim CEO – January 2026
- Topline release from CRDF-004 – January 27th, 2026
- Meetings with FDA for Phase III trial design – 1H:26
- Additional data release from CRDF-004 – 2Q:26
- Launch of Phase III onvansertib trial (CRDF-005) - 2026
Summary
Cardiff presented topline data from its Phase II CRDF-004 study which supports the use of the 30 mg dose of onvansertib in the anticipated Phase III trial that will be designed with the FDA in the coming months. The report of data coincides with the departure of CEO Mark Erlander and CFO James Levine and their replacement with CEO Mani Mohindru and Senior Vice President of Finance Brigitte Lindsay. It was unclear what precipitated the change; however, Dr. Mohindru has a deep understanding of the company and her role having served on its board since 2021, and experience in other executive positions. She is serving in an interim capacity while the board conducts a search to identify a new CEO and CFO.
Data from the CRDF-004 trial highlighted a favorable ORR and hazard ratio for the 30 mg onvansertib arm. Median PFS has not yet been reached. An important early indicator, PFS at six months, was more than five percentage points better at 94.1%. While the data were positive, the trial is still ongoing, and there is other data that will further clarify the safety and efficacy of the regimen. Management has indicated that they expect to move forward with the Phase III study using onvansertib + FOLFIRI/bev to the combined SoC arm following a meeting with the FDA.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
________________________
[1] FOLFIRI is a chemotherapy regimen commonly used to treat colorectal cancer: FOL - Folinic acid (leucovorin) F - 5-fluorouracil (5-FU) IRI - Irinotecan
[2] FOLFOX: FOL - Folinic acid (leucovorin) F - 5-fluorouracil (5-FU) OX - Oxaliplatin
[3] FOLFOX and FOLFIRI are often considered equivalent first-line options for metastatic colorectal cancer. The choice between them may depend on the patient's tolerance for specific side effects (neuropathy and diarrhea), previous treatments received, overall health status and tumor characteristics.
[4] Using Fisher’s Exact Test.
[5] In survival analysis, the hazard ratio (HR) is the ratio of the hazard rates corresponding to the conditions characterized by two distinct levels of a treatment variable of interest. For example, in a clinical study of a drug, the treated population may die at half the rate of the control population. The hazard ratio would be 0.5, indicating a lower hazard of death from the treatment.
[6] Using Log-rank Test
[7] Data cut-off as of January 22nd, 2026. See exhibit in the press release for footnotes and explanations. Chart included in this report for summary purposes only.