By David Bautz, PhD
NASDAQ:FBLG
READ THE FULL FBLG RESEARCH REPORT
Business Update
Approval from HREC for Phase 1/2 Diabetic Foot Ulcer Trial
In November 2025, FibroBiologics, Inc. (NASDAQ:FBLG) announced that it has received both public and private Human Research Ethics Committee (HREC) approvals in Australia for the planned Phase 1/2 clinical trial of CYWC628 for the treatment of refractory Diabetic Foot Ulcers (DFU). The approval allows for enrollment of 120 patients across 10 clinical sites in Australia. Now that HREC approval has been granted, the company has fulfilled all necessary regulatory approvals and required filings prior to proceeding with the clinical trial, which we anticipate initiating in the first quarter of 2026.
The trial is a multicenter, randomized study designed to evaluate the safety, tolerability, and efficacy of FibroBiologics’ topically administered allogenic fibroblast therapy, CYWC628. Study subjects will receive up to 12 weeks of treatment using either standard of care (SoC) plus a high or low dose of CYWC628, or SoC alone. Study outcomes include wound healing, efficacy of response, and safety parameters. An interim analysis will be conducted after a predefined number of participants complete six weeks of treatment to assess primary safety and efficacy endpoints. The results from the interim analysis could be available in mid-2026, with topline results expected before the end of 2026.
DFUs cause significant morbidity for the 6.3% of diabetic adults (~33 million) that develop them. Of those, 20% will require lower extremity amputation, and 10% will die within the first year of their first DFU. In addition, once a DFU forms, there is a high rate of recurrence, both at one year (40%) and three years (70%).
Fibroblasts have excellent therapeutic potential in the treatment of DFUs due to the critical role they play in every stage of wound healing, including hemostasis, inflammation, proliferation, and remodeling. Importantly, fibroblasts are the key cells that secrete extracellular matrix proteins that maintain all the tissues and organs in the body.
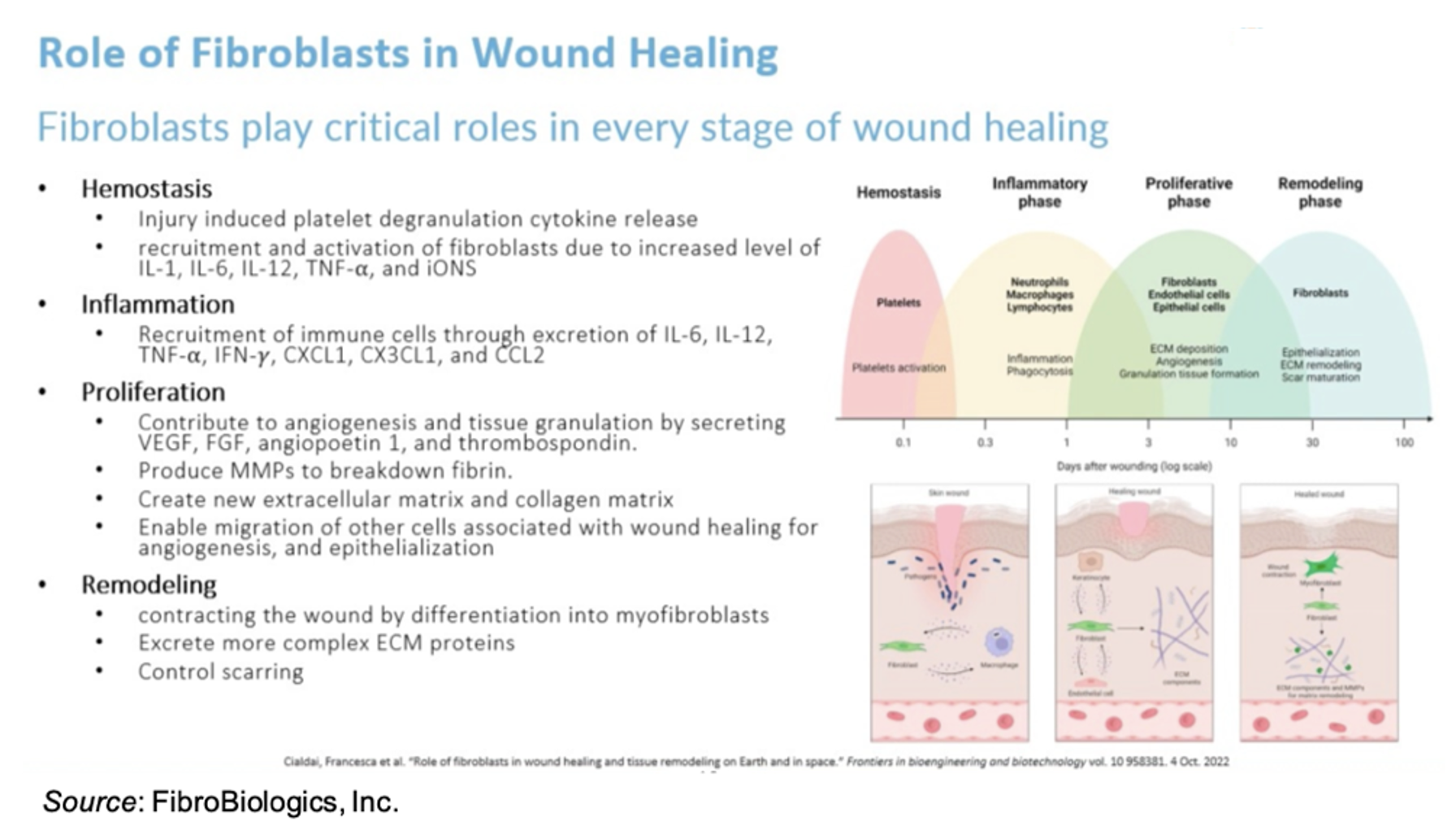
The company does not utilize single cell fibroblasts for treatment but instead a fibroblast spheroid, which is composed of approximately 3,000 fibroblasts and is administered to the top of the wound, at which time the cells migrate from the surface of the wound and release various cytokines and growth factors to initiate the wound healing process. The use of spheroids is more practical from a therapeutic perspective as they have higher viability than single cells, they don’t require pre-culturing before administration, they can be easily frozen and thawed, and they have a significantly higher potency and efficacy compared to single cells.
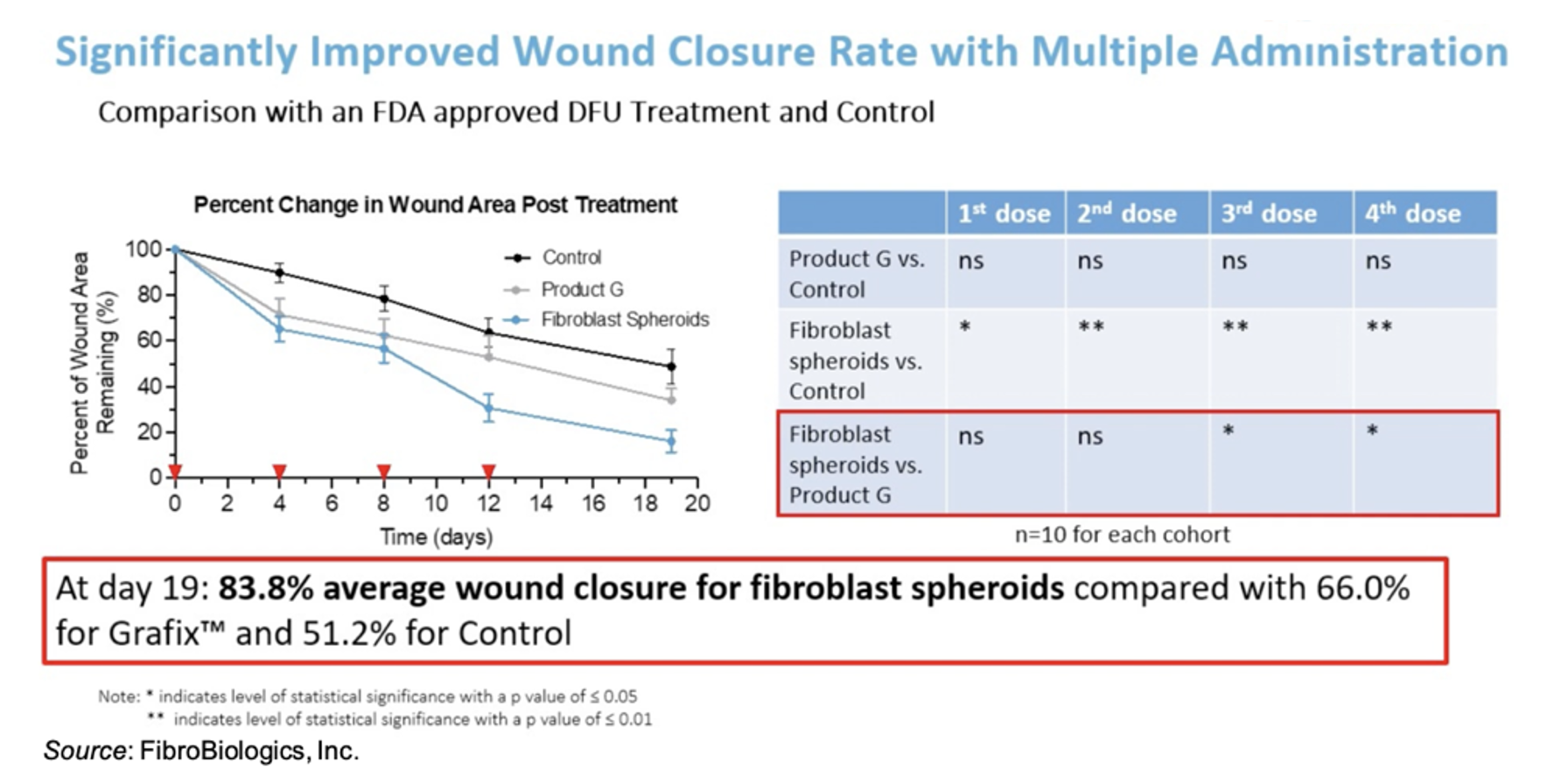
FibroBiologics has compiled a robust pre-clinical data set showing the efficacy of fibroblast spheroids in the treatment of wounds. For example, the following figure shows results using a diabetic mouse model in which administration of fibroblasts led to a statistically significant average 83.8% wound closure by Day 19 compared to 66.0% for Grafix™ and only 51.2% for control.
While wound healing is important, the quality of the wound healing is just as important. FibroBiologics has data on seven key biomarkers that are key to demonstrating the quality of the wound healing. Fibroblast treatment shows much better re-epithelialization, granulation, cell proliferation, neo-vascularization, recruitment and proliferation of fibroblasts, keratinocyte migration, and epithelial-mesenchymal transition. Intriguingly, even though the fibroblast spheroids are administered topically, there appears to be a systemic effect on cytokine levels, including IL-6, TNF-α, IL-1β, and IL-10.
The use of fibroblast spheroids for wound healing can be thought of as a platform technology. In addition to the treatment of DFUs, fibroblast spheroids could also be used for the treatment of burns and surgical wounds.
In regard to safety, the company has performed a number of experiments to examine any potential adverse events associated with fibroblast-based therapy. The cells do not graft into tissue. Following application, the cells stay on the surface of the wound and initiate the healing process before gradually dying off within four days of treatment. In addition, there is no impact on CBC, WBC, liver function, or kidney function, thus showing that administration of fibroblasts appears to be quite safe.
Positive Preclinical Results in Degenerative Disc Disease Animal Model
On January 5, 2026, FibroBiologics announced positive preclinical results for a Fibroblast Spheroid-derived Chondrocyte (FSdC) therapy that showed superior improvement in recovering intervertebral disc integrity and preventing degeneration in animal models of degenerative disc disease. The condition affects millions of adults in the United States and is a leading cause of chronic back pain and disability.
In the current studies, the efficacy of FSdC spheroids was compared to 2D fibroblasts and fibroblast spheroids. The data showed that FSdC spheroids achieved the most statistically significant difference from control with a P value of 0.00015, compared to a P value of 0.036 for 2D fibroblasts and 0.068 for fibroblast spheroids. FSdC spheroids also showed the highest level of intervertebral disc height recovery after 12 weeks of treatment compared to the other two fibroblast therapies. These results suggest FSdC spheroids may offer disease-modifying effects and not just alleviation of symptoms, based on their ability to sustain the highest average disc size index across all time points. In addition, these results de-risk the platform as it advances toward clinical trials and supports an additional pipeline indication for the company’s fibroblast-based technology.
IND Application Filed for CYPS317 in Psoriasis
On December 31, 2025, FibroBiologics announced the filing of an Investigational New Drug (IND) application with the U.S. FDA seeking regulatory clearance to initiate clinical trials of CYP371 for the treatment of moderate to severe psoriasis. This filing is based in part on positive IND-enabling preclinical results demonstrating the potential for fibroblast spheroids to significantly reduce psoriasis disease severity and relapse in preclinical models. For example, a single dose of CYPS317 was comparable or better to multiple doses of an anti-IL-23 monoclonal antibody and yielded significant reduction in disease recurrence. Following regulatory clearance of the IND, FibroBiologics intends to pursue a clinical development program for CYPS317 with a near-term goal of initiating first-in-human trials.
Financial Update
During November and December 2025, FibroBiologics raised gross proceeds of approximately $7.2 million:
- On November 19, 2025, the company raised approximately $4 million from the sale of 3.54 million shares of its common stock and pre-funded warrants to purchase approximately 8.57 million shares of common stock at a purchase price of $0.3303 per share. In addition, the company issued and sold warrants to purchase one share of common stock for each share of common stock or pre-funded warrant purchased in the offering, for up to 12,110,203 shares of common stock. The warrants have an exercise price of $0.3303 per share, will be exercisable beginning on the date of approval by company stockholders to issue the shares upon exercise of the warrants and will expire five years following the date of stockholder approval.
- On November 25, 2025, the company raised approximately $1.5 million in gross proceeds from the sale of 4,477,614 shares of its common stock at a price of $0.335 per share. In addition, the company issued and sold warrants to purchase 4,477,614 shares of common stock at an exercise price of $0.335 per share. The warrants will be exercisable beginning on the date of approval by company stockholders to issue the shares upon exercise of the warrants and will expire five years following the date of stockholder approval.
- On December 16, 2025, the company raised approximately $1.7 million in gross proceeds from the sale of 5,227,275 shares of its common stock at an offering price of $0.33 per share. In addition, the company issued and sold warrants to purchase 5,227,275 shares of common stock at an exercise price of $0.33 per share. The warrants will be exercisable beginning on the date of approval by company stockholders to issue the shares upon exercise of the warrants and will expire five years following the date of stockholder approval.
In November 2025, FibroBiologics announced it has paid all amounts outstanding under the convertible promissory notes issued to YA II PN, Ltd., an investment fund managed by Yorkville Advisors Global, LP pursuant to the Standby Equity Purchase Agreement (SEPA) entered into in December 2024 between the company and Yorkville. Yorkville advanced $15 million (of up to $25 million total under the SEPA) to the company in three equal tranches of Notes. The Notes are no longer outstanding and there will be no further conversions into shares of the company’s common stock. The SEPA remains in place until Dec. 20, 2026 and it allows FibroBiologics to sell, at its discretion, an additional $10 million in common stock to Yorkville.
Conclusion
FibroBiologics continues to execute on its business plan with the expected initiation of the Phase 1/2 clinical trial of CYWC628 in the first quarter of 2026, the IND filing for CYPS317 for the treatment of psoriasis, the recent financings, and paying off all outstanding debt to clean up its balance sheet. We look forward to results from the CYWC628 trial, with interim results possible in mid-2026 and topline results expected before the end of the year. After incorporating the recent financings into our model, our valuation is now at $6.50 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.