By John Vandermosten, CFA
NYSE:PLX
READ THE FULL PLX RESEARCH REPORT
CHMP Positive Opinion for Elfabrio Four-Week Dosing
In October 2025, Protalix BioTherapeutics, Inc. (NYSE:PLX) announced that the Committee for Medicinal Products for Human Use (CHMP) issued a negative opinion regarding Chiesi’s request for four-week dosing via a post-authorization variation. In response, Chiesi and Protalix requested a re-examination and new CHMP recommendation. In January, the European agency reversed its prior conclusion following the appeal. The committee issued a positive opinion recommending approval of the 2.0 mg/kg every four weeks dosing regimen for Elfabrio in Fabry disease adult patients stable with an enzyme replacement therapy (ERT) treatment.
The data to support the extension of time between infusions was generated in the Phase III BRIGHT study. Elfabrio has a prolonged half-life, which enables the dosing period to be extended. Adults with Fabry disease already stable on biweekly ERT (agalsidase alfa or beta) for more than three years switched to intravenous pegunigalsidase alfa (Elfabrio) 2.0 mg/kg every 4 weeks for 52 weeks. Kidney function in the stable ERT-experienced group was maintained over a year. There was also an extension to the BRIGHT study, which allowed patients to continue on this regimen. Longer term data from the extension group demonstrated that the change did not increase immunogenicity or create new administration risks.
The next step in the process is for the European Commission (EC) to decide whether or not to approve the abbreviated dosing schedule recommended by the CHMP. If approved, Protalix will be eligible to receive a regulatory milestone payment of $25 million. We do not include the milestone in our revenue estimates.
The three approved ERTs (Fabrazyme, Replagal, and Elfabrio) all require an intravenous (IV) infusion every two weeks, which is a burden that can be reduced by doubling the time between infusions. The change can also reduce cost where a provider administers the infusion. Other benefits include less venous access trauma, easier scheduling, and a higher quality of life for the Fabry patient. Chiesi and Protalix management anticipate that the EC will issue a decision by March 2026.
Background on CHMP Opinion
In December 2024, Protalix’s partner Chiesi submitted a Variation Application to the EMA that requested a change in the dosing regimen for Elfabrio. Based in part on the findings in the BRIGHT study and on new pharmacokinetic data, the sponsors sought a less frequent dosing regimen at a dose of 2 mg/kg body weight administered every four weeks in adult patients with Fabry disease in the European Union. Analysis of the BRIGHT study concluded that treatment with Elfabrio every four weeks could offer a new treatment option for patients with Fabry disease.[1]
On October 17th, 2025, Chiesi and Protalix announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) had issued a negative opinion on the request to approve the dosing regimen of 2.0 mg/kg body weight infused every 4 weeks for Elfabrio.
Two and a half weeks after the negative opinion, Chiesi and Protalix issued a press release stating that they would seek re-examination of the EMA’s negative opinion for Elfabrio regarding the four-week alternative dosing regimen. The process requires that the sponsor submit a written notice to the EMA within 15 days of the CHMP opinion and 60 days later submit the grounds for examination. A different rapporteur and co-rapporteur will be appointed to conduct the re-examination. Chiesi and Protalix have consultants and/or internal personnel with EMA and CHMP experience who will help develop the argument for four-week dosing. In the meantime, two-week dosing remains approved and the standard for administering Elfabrio.
Letter to Stockholders
In the first days of 2026, Protalix CEO Dror Bashan penned a letter to stockholders highlighting the company’s accomplishments in the prior year and looking ahead to future sales mix and revenue trends. Along with two commercial assets, Protalix has one clinical program, another on the cusp of an investigational new drug (IND) application, and other assets in discovery. The company’s priorities emphasize the relationship with Chiesi for commercialization of Elfabrio, support the advancement of PRX-115 into a Phase II study and beyond if appropriate, and further development of the rare renal disease programs.
The lead development program, PRX-115, has shown a rapid and durable urate-lowering effect with a favorable tolerability profile in a Phase I study in gout. It is pursuing an indication that is increasing in prevalence, and with many patients suffering from uncontrolled disease. Management believes that PRX-115 has the potential to deliver a differentiated clinical profile with rapid onset and durable urate control and potentially emerge as a third molecule for commercialization.
As mentioned in our previous report, Protalix announced a collaboration with the Germany-based Secarna Pharmaceuticals to develop novel antisense oligonucleotide (ASO) therapies using Secarna’s OligoCreator platform. The arrangement will seek pharmaceutical candidates for rare renal indications. Details of the arrangement were provided in a December 17th press release.
Secarna Pharmaceuticals is an artificial intelligence (AI)-powered therapeutics development company with two platforms and a pipeline of assets focused on discovery and investigational new drug (IND)-enabling studies. It has several partners, including Lipigon Pharmaceuticals, Denali Therapeutics, Curie Bio, SciNeuro Pharmaceuticals, and Evotec/Bristol Myers Squibb that are developing their own products using Secarna’s platforms. The most advanced of the partner projects is Lipigon’s Phase II Lipisense asset.
Pipeline
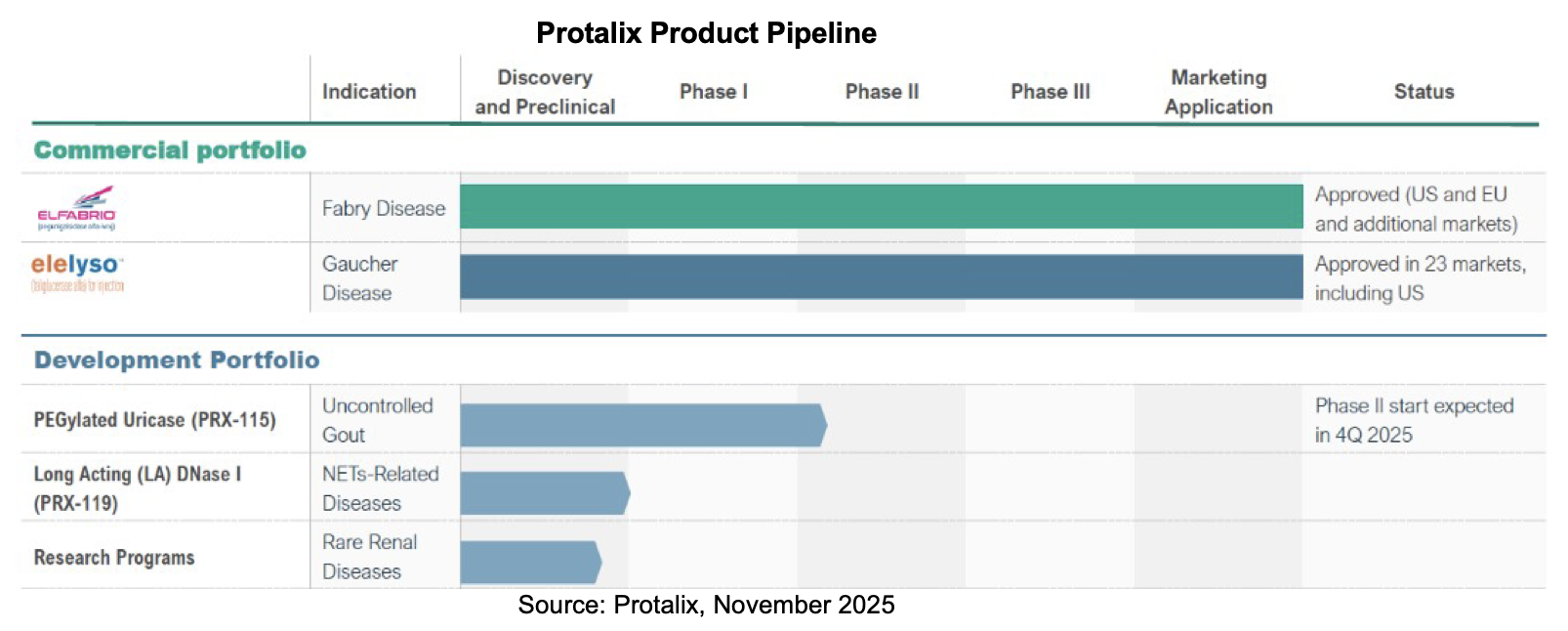
Milestones
- Appointment of Gilad Mamlok as CFO – August 2025
- Participation at HC Wainwright Global Investment Conference – September 2025
- CHMP issued negative opinion of Elfabrio four-week dosing – October 2025
- Automatic 5-year extension of Pfizer-Elelyso contract to 2030 – October 2025
- Protalix & Chiesi appeal CHMP decision – November 2025
- PRX-115 IND becomes effective – November 2025
- Ongoing enrollment in Japanese RISE study (Elfabrio) - 2025
- Pediatric FLY study active for Fabry disease (Elfabrio) - 2025
- Initiate Phase II study for PRX-115 in gout – 2H:25
- Collaboration with Secarna Pharmaceuticals in renal rare disease – December 2025
- Enrollment of first patient in PRX-115 Phase II gout study – 4Q:25
- Positive opinion from CHMP for Elfabrio four-week dosing – January 2026
- PRX-115 Phase II trial start – 1Q:26
- EC decision for Elfabrio four-week dosing – March 2026
- Topline results from PRX-115 Phase II study - 2027
Summary
Protalix announces good news with the positive opinion from the CHMP. The opinion was the result of Chiesi’s request for re-examination of data from Protalix’s BRIGHT study. BRIGHT examined the long-term effects of a 2.0 mg/kg dose administered every four weeks of Elfabrio. The CHMP will pass the application package on to the EC who has about two months to review. Based on management commentary and a review of other literature on the topic, in most cases the EC follows the CHMP opinion. We expect that the EC will approve the new regimen, which will entitle Protalix to a $25 million milestone payment. We have not included this contribution in our model.
Prior to the positive CHMP announcement, CEO Dror Bashan wrote a letter to investors highlighting the company’s commercial and development prospects. Growing revenues for Elfabrio and Elelyso join pipeline candidates, including PRX-119 for NETs-related diseases and PRX-115 for uncontrolled gout. The Phase II PRX-115 trial is expected to start soon. While the revenue profile for both assets is volatile, we think that Elfabrio revenues have substantial upside that will be clearer after initial inventory for each of the regions is consumed, and patient demand patterns can be predicted. Our valuation remains at $10 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
________________________
[1] Holida, M., et al. A phase III, open-label clinical trial evaluating pegunigalsidase alfa administered every 4 weeks in adults with Fabry disease previously treated with other enzyme replacement therapies, Journal of Inherited Metabolic Disease. October 2024.