By John Vandermosten, CFA
NASDAQ:RADX
READ THE FULL RADX RESEARCH REPORT
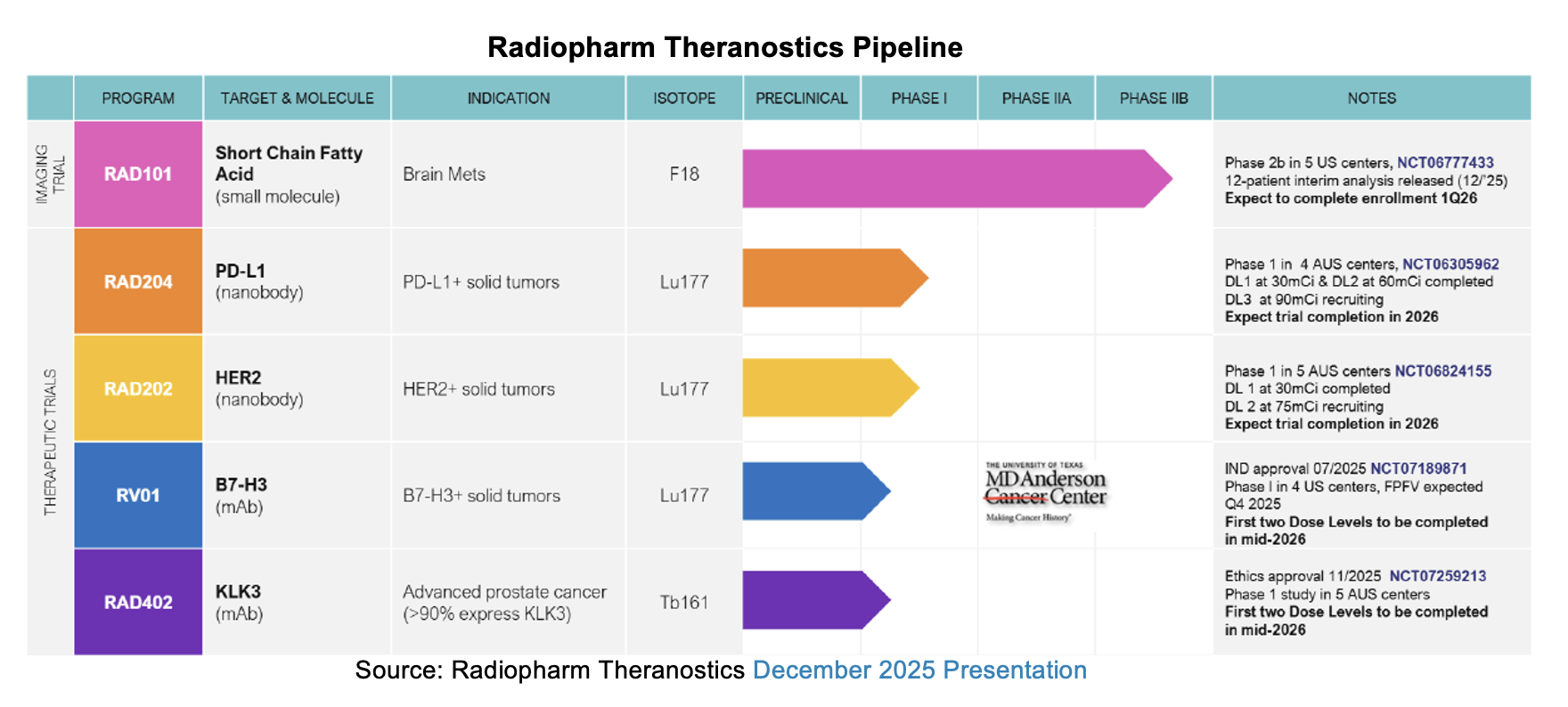
Radiopharm Theranostics (NASDAQ:RADX) released its first half 2026 cash flow statement on January 28th, 2026. The company has a June 30 fiscal year end and reports audited financial statements semiannually. We expect the half year report, prepared under International Financial Reporting Standards (IFRS), to be issued next month. The period captured in this report is from July 1st to December 31st 2025. Beyond the review of cash sources and uses for the first half of fiscal year 2026, the report summarizes the status of each of the company’s pipeline assets. Since the previous financial update, Radiopharm has presented interim data for its RAD101 imaging agent and reiterated upcoming milestones for RAD202 and RAD204, and anticipated delivery of data.
Radiopharm’s operating activities consumed ($22.7) million for the six-month period ending December 31st, 2025. Cash from financing activities was $33.4 million for the same six-month period.[1]
For the first six months of the reporting period ending December 31st, 2025:
- There were no cash receipts from customers;
- Research and development consumed ($19.4) million related to the management of multiple clinical trials;
- Advertising and marketing costs consumed ($228,000);
- Staff costs consumed ($6.3) million;
- Administration and corporate costs were ($1.9) million;
- Other miscellaneous cash operating contributions total $5.1 million, mostly impacted by $4.5 million in government grants and tax incentives;
- Other payments categorized as investing activities consumed ($5.3) million for payments of license fees;
- Financing cash was $33.4 million from the issuance of equity securities, partially offset by transaction costs.
As of December 31st, 2025, Radiopharm held $34.5 million in cash compared to $19.0 million at the end of FY:25. Cash burn for 1H:26 was ($28.0) million.
RAD101 Phase IIb Interim Analysis
Radiopharm Theranostics reported the primary endpoint for its RAD101 trial in an interim analysis on December 15th. Following the release, management held a webcast which featured the company’s CEO, Riccardo Canevari, Chief Medical Officer Dr. Dimitris Voliotis, and the principal investigator on the RAD101 trial, Dr. Harshad Kulkarni.
Topline from the release indicated that 92% (11/12) of evaluated patients treated with RAD101 achieved concordance with Magnetic Resonance Imaging (MRI) imaging, which was the primary endpoint. RAD101 uptake was selective and significant in suspected or recurrent brain metastases.
RAD202
RAD202, a Lu-177 bound nanobody targeting HER2, is being evaluated in the active HEAT trial for treatment of patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive advanced solid tumors. RAD202 was cleared to move to the next dose level of 75 mCi by the Data Safety and Monitoring Committee (DSMC). The study has completed dosing of the 30 mCi cohort and data from the first three patients in this cohort show significant tumor uptake in HER2 positive tumors. The principal investigators observed a favorable safety profile with no drug-related adverse events reported.
RAD204
RAD204 is a Lu-177 bound nanobody targeting PD-L1 being evaluated for treatment of non-small cell lung cancer (NSCLC), small-cell lung cancer (SCLC), triple-negative breast cancer (TNBC), cutaneous melanoma, head and neck squamous cell carcinoma (HNSCC), and endometrial cancer. The first and second cohorts of the Phase I study are complete, and the third cohort is cleared to start at a 90 mCi dose of Lu-177. In the 30 mCi cohort, two of three patients achieved stable disease for 5.5 months compared to standard of care at 3.5 months. Tumor uptake for RAD204 in the first six patients in cohorts one and two shows results in line with previous imaging studies with the antibody. Investigators observe a reassuring safety profile, reporting no drug-related adverse events.
RV01
RV01, also known as Betabart, is a monoclonal antibody targeting the 4Ig isoform of B7-H3. B7-H3 is highly expressed in a variety of tumors. RV01 is the subject of a joint venture between Radiopharm and MD Anderson Cancer Center. In January 2026, Radiopharm increased its ownership in the JV to 87.5% to reflect increasing interest in the asset. Last July, the FDA cleared RV01’s investigational new drug (IND) application, readying the candidate to begin a Phase I trial. Radiopharm expects to dose the first patients in 1Q:26.
RV01 has a competitor in the B7-H3 targeting arena. Aktis Oncology (AKTS) recently held an initial public offering last month raising more than $365 million. A portion of these funds will be allocated to a Phase Ib trial for AKY-2519, which is an Ac-225 miniprotein radioconjugate targeting B7-H3. The marker is expressed in a wide variety of cancers, including colorectal, prostate, breast, and others.[2] A notable difference between the two candidates is the radioisotope used. While RV01 uses a Lutetium-177 radionuclide, which emits beta and gamma particles, AKY-2519 employs the alpha emitter Ac-225. Beta radiation has a low linear energy transfer with a medium sphere of impact, with the benefit of killing nearby tumor cells that do not express B7-H3. Alpha emitters are high-energy, short-range particles that cause double-strand DNA breaks and are more appropriate for precision treatment.
AKY-2519 is now conducting investigational new drug (IND) enabling studies, while RV01 is the subject of a Phase I study. We anticipate that RV01 will dose its first patients in the next few weeks, giving a slight edge to Radiopharm on timing.
RAD402
RAD402 binds a kallikrein-related peptidase 3 (KLK3) targeting antibody to a Terbium 161 isotope for treating prostate cancer. It will soon advance from the preclinical stage, where it demonstrated strong tumor targeting, limited bone marrow uptake, and a hepatic excretion profile consistent with other monoclonal antibodies in murine xenografts. Last November, RAD402 was granted clearance in Australia to begin a Phase I study for treatment of metastatic or locally advanced prostate cancer. The trial is expected to begin in 1Q:26.
RAD301
RAD301 is the subject of a Phase I imaging trial in pancreatic ductal adenocarcinoma (PDAC). It is a Ga-68 radionuclide bound to an antibody targeting αvB-integrin. αvB-integrin is a cellular marker for tumor invasion and metastatic growth, which correlates with decreased survival in several carcinomas, particularly pancreatic. The candidate has been awarded an orphan drug designation by the FDA.
Corporate Milestones[3]
- Oliver Sartor appointed to Scientific Advisory Board – July 2025
- FDA clears IND for Phase I RV01 study – July 2025
- Request for ethics approval for Phase I RAD402 trial - 3Q:25
- Filing of FY:25 Annual Report – September 2025
- RAD204 data from first two cohorts – 2H:25
- RAD101 patient recruitment – 2H:25
- Launch of Phase I RV01 (Betabart) trial – 4Q:25
- Begin dosing patients in Phase I RAD 402 trial – 4Q:25
- RAD301 Phase I last patient dosed – 4Q:25
- RAD202 report data from first two cohorts - end of 2025
- Dosing of first patients in RV01 trial – 1Q:26
- RAD101 Phase III trial launch – 2H:26
- RAD101 Phase II trial fully enrolled – February 2026
- RAD402 Phase I trial initiation in metastatic or locally advanced prostate cancer – 1Q:26
- RAD101 Phase II readout – 1H:26
- RAD202 Phase I data release (2 cohorts) – 1H:26
- RAD204 Phase I dose escalation complete – mid-2026
- RAD202 Phase I last patient dosed – 2H:26
- RAD301 Phase II trial start – 2H:26
- RAD101 Phase III launch – 2H:26
- RAD204 start Phase II study - 2027
- RAD301 Phase II trial complete – 2H:27
- RAD204 complete Phase II study – 4Q:27
- RAD101 NDA submission - 2028
Summary
Radiopharm issues its 1H:26 cash flow report and updates investors on the status of its pipeline programs. Just seven weeks ago, Radiopharm provided an interim look at its Phase IIb RAD101 trial, generating impressive results for its primary endpoint. Results demonstrated a 92% concordance between F-18 pivalate measured by PET and MRI for the first twelve patients in its trial. While not final data, these results in 11 of 12 patients show that RAD101 in PET scans provides valuable information to oncologists that can be used to determine optimal treatment. In many cases, results from MRI are inconclusive and may delay necessary treatment or lead to further and unnecessary SRS treatment. As for next milestones, we expect to see a further readout for RAD101 in 1H:26 and the launch of a Phase III study in 2H:26. We also expect to see interim data from the Phase I RAD202 and RAD204 studies in the middle of 2026.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives payments totaling a maximum fee of up to $50,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
________________________
[1] Note that results are reported in Australian Dollars. The most recent exchange rate between Australian Dollars and U.S. Dollars is $1.44 AUD to $1.00 USD.
[2] The Human Protein Atlas. CD276 (B7-H3). Expression in cancer.
[3] Quarters and halves listed in the milestones section are calendar quarters and halves in contrast to Radiopharm’s June 30 fiscal year end.