By M. Marin
NASDAQ:BMRA
READ THE FULL BMRA RESEARCH REPORT
COVID-19 Test Contributes to More Than Doubling Of Revenue in 3Q FY21
Biomerica (NASDAQ:BMRA) recently reported Q3 fiscal 2021 results. BMRA’s focus in 2020-21 has included extending its expertise and technology to develop and commercialize COVID-19 diagnostics.
Revenue in 3Q FY 2021 more than doubled to $3.62 million, largely the result of initial sales of the company’s new COVID-19 antigen rapid test in Europe after BMRA received CE Mark approval in January 2021. We believe this both creates a new revenue stream and also offers proof-of-concept of the versatility and applicability of the company’s technology and IP.
Planning to Expand Markets For COVID-19 Test
BMRA has been shipping the test in the EU, as noted, leveraging its existing distribution network. BMRA is optimistic about sales of the COVID-19 test and is working to expand into additional markets. We believe the global need to test for the virus is likely to persist even with rising vaccination rates. The FDA is reviewing the company’s EUA submission for U.S. sales.
Measures to Strengthen Balance Sheet
The company also took measures to simplify and strengthen its balance sheet in 3Q FY 2021. Following the conversion of preferred shares in January, BMRA now has no preferred shares outstanding. BMRA also issued 158,889 shares under its shelf registration, raising net proceeds of about $1.0 million. The company ended 3Q with cash & equivalents of $5.27 million to continue advancing its InFoods platform and support overall growth.
InFoods Study Moving Forward; Data Expected in 2H 2021
BMRA continues to advance the InFoods clinical trial. The company anticipates that results from the endpoint trial will lead to partnership discussions with larger market participants and also advance the inFoods path to potential FDA clearance. The total direct and indirect cost of IBS has been estimated at $30 billion annually in the U.S. market alone. As a diagnostic-guided therapy (not a drug), InFoods does not have the side effects drugs often have and can be used alone and/or as part of a drug therapy program.
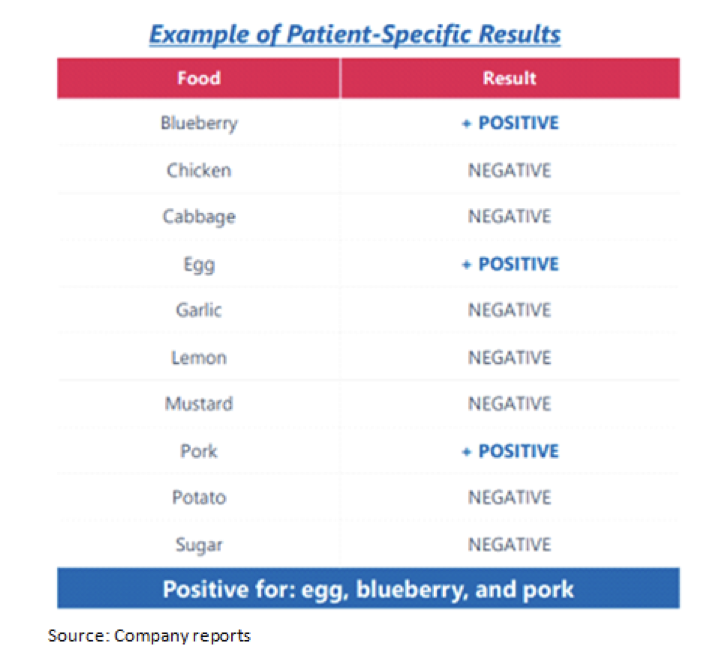
IBS symptoms frequently are triggered when people eat specific foods. Trigger foods vary from patient-to-patient and are specific for each individual patient. For instance, one person might be able to consume broccoli with no problem but milk or eggs trigger discomfort and symptoms, while another person might be fine with milk but cannot tolerate broccoli. Eliminating trigger foods from the patient’s diet is expected to alleviate IBS symptoms, discomfort and pain. Utilizing an antibody-guided blood test, the InFoods® technology identifies foods that trigger IBS symptoms and pain in order to help create a dietary regime designed to minimize ongoing occurrence of symptoms.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.