By David Bautz, PhD
NASDAQ:CFRX
READ THE FULL CFRX RESEARCH REPORT
Business Update
Rapid Resolution of Symptoms in Phase 2 Trial
On October 4, 2021, ContraFect Corp. (NASDAQ:CFRX) announced new data from the company’s Phase 2 clinical trial of exebacase showing rapid symptom resolution in patients with Staphylococcus aureus bacteremia was presented as a Late Breaker oral presentation at IDWeek™ 2021.
The Phase 2 study was an international, multicenter, randomized, double blind, placebo controlled trial with a superiority comparison between exebacase or placebo combined with the standard of care antibiotics. A total of 121 patients were randomized 3:2 to receive a single dose of 0.25 mg/kg exebacase or placebo administered via a two-hour infusion along with the standard of care antibiotics. The primary endpoint of the study was early clinical response. A patient was considered a responder if they were alive, had an improvement or resolution of the signs and symptoms attributable to the S. aureus infection, there were no additional medical interventions required, and there was no evidence of a spread of the infection. Please see our previous reports (here and here) for an overview of results from the trial.
The new data presented at IDWeek™ 2021 concerned symptom resolution in the modified intent-to-treat (mITT) population (n=71 exebacase+SOC; n=45 SOC alone). The results showed that 86 patients with S. aureus bacteremia had at least one symptom at baseline (n=53 exebacase+SOC; n=33 SOC alone). The symptoms resolved in the majority of these patients (94.3% exebacase+SOC; 87.9% SOC alone). The percentage of patients whose symptoms resolved was similar for those with methicillin-resistant S. aureus (MRSA) infections as well (94.4% exebacase+SOC; 81.8% SOC alone). Where exebacase differentiated itself was in the median time to resolution. For the mITT group, the median time to symptom resolution for the exebacase+SOC group was 3 days (95% CI 3-7) compared with 6 days (95% CI 3-7) for the SOC alone group. Similar results were seen for the MRSA subgroup. The results are summarized in the following table.
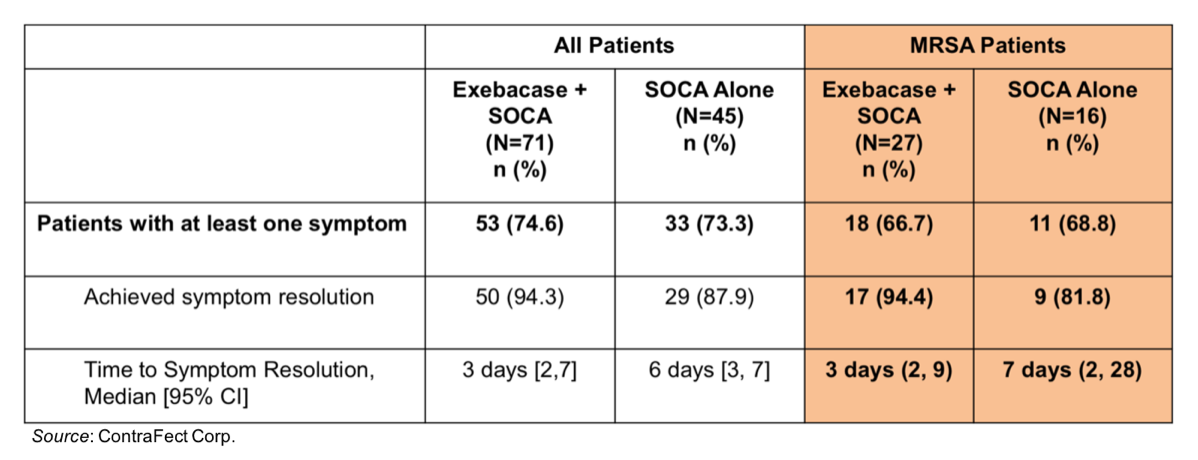
Coupled with the previously disclosed data from the trial showing a 42.8% higher clinical responder rate at Day 14 with exebacase in the prespecified MRSA subgroup, the newly presented data on reduction in the median time to symptom resolution shows that rapid bacteriolysis caused by exebacase may translate into a clinical benefit for patients with S. aureus bacteremia.
In Vitro Activity of Exebacase Against S. Aureus Clinical Isolates
In addition to the oral presentation at IDWeek™ 2021, ContraFect also presented a poster on the in vitro activity of exebacase against clinical isolates of S. aureus collected during the COVID-19 pandemic. This work was done as part of the SENTRY Antimicrobial Surveillance Program. A total of 2,849 pathogens were collected from blood cultures of patients suffering from bloodstream infections at 29 U.S. medical centers (20 states) during 2020. Of the 2,849 pathogens collected, 666 (23.4%) were identified as S. aureus and included in the study. This is in line with the previous five years showing S. aureus represented approximately 24% of the causative pathogens of bloodstream infections dating back to 2016. The following table shows the minimum inhibitory concentration (MIC) distribution for the 666 S. aureus isolates. Exebacase inhibited 100% of S. aureus isolates at MIC values ≤ 1 μg/mL and showed equivalent MIC results for both MSSA and MRSA strains.
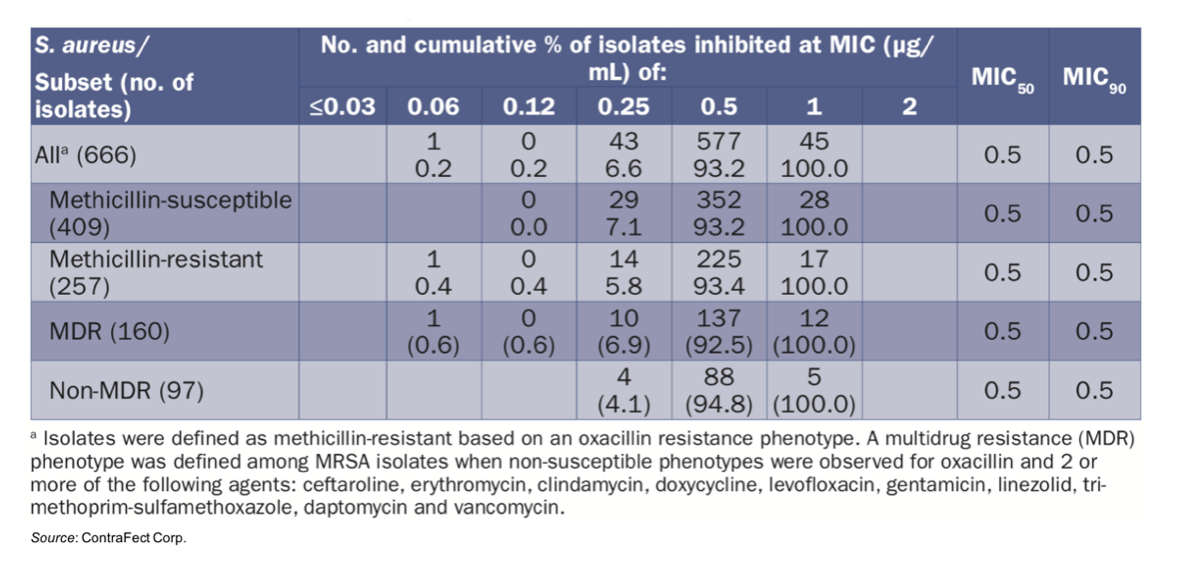
A series of tests were performed with comparator agents (antibiotics), with most being active (91.7% - 100% susceptible) against the MSSA population, while many had reduced susceptibility against MRSA, including ceftaroline (88.3% susceptible). However, drugs utilized for treating MRSA infections (daptomycin and vancomycin) were active (100% susceptibility) against all isolates.
Of the MRSA isolates, 62.3% of them were classified as multi-drug resistant (MDR). Importantly, exebacase showed equal MIC50 and MIC90 against the MDR and non-MDR strains (MIC50/90, 0.5/0.5 μg/mL). Daptomycin and vancomycin were also active (100% susceptible) against the MDR strains.
These results show that there was not much impact on the etiology of bloodstream infections due to the COVID-19 pandemic and that the occurrence of MRSA infections was actually slightly lower in 2020 (38.6%) compared to previous years (39.5% - 43.1%). It is good to see that exebacase continues to be active against clinical isolates, regardless of resistance phenotype, particularly since the study included clinical isolates recovered from patients that are reflective of the patient cohort in the company’s ongoing Phase 3 clinical trial.
Conclusion
The data presented by ContraFect at IDWeek™ 2021 support the continued development of exebacase as a treatment for bloodstream infections caused by S. aureus. The ongoing Phase 3 DISRUPT (Direct Lysis of Staph aureus Resistant Pathogen Trial) trial is continuing to enroll patients and we look forward to an update from the company on when the preplanned interim futility analysis will take place, which is scheduled to occur after the first 60% of patients are enrolled in the trial and evaluable for efficacy. With no changes to our model our valuation remains at $23 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.